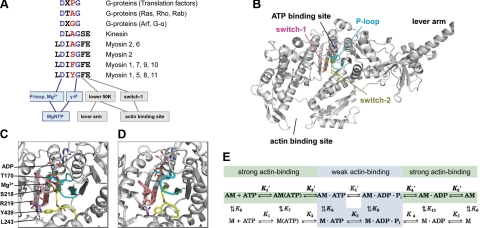
Figure 1.
Sequence, location, and roles of switch-2. A) Switch-2 amino acid sequence and conserved communication pathways in different NTPases. Residues and pathways conserved in myosins, kinesins, and G proteins are colored blue. Class-specific residue mutated in the current study is highlighted in red. B) Location of conserved loops of the ATPase active site within the motor domain of myosin-5a (1W7J). C, D) Magnified views of the active site in strongly (1W7J, C) and weakly (1W7I, D) nucleotide-bound states. E) Kinetic scheme of the actomyosin ATPase cycle. Nucleotide-free (rigor) actomyosin (AM) binds ATP in two steps (K1′ and K2′). Myosin head (M) then dissociates (K8) from actin (A) in the main flux pathway indicated by shading and bold characters. After ATP hydrolysis (K3), M · ADP · Pi rebinds to actin (K9), and products are released (Pi in K4′ and ADP in K5′). Strong and weak actin-binding states are shaded in green and cyan, respectively. All rate and equilibrium constants are defined in the rightward and downward directions.