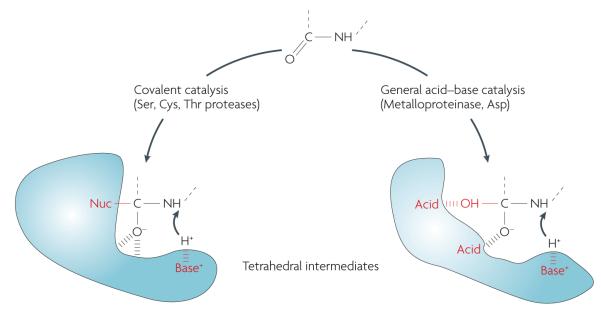
Figure 1. Tetrahedral intermediates formed during peptide cleavage by proteases.
All proteases accelerate the formation of a transition state in peptide-bond hydrolysis. This state, termed the tetrahedral intermediate, is a prerequisite for peptide-bond scission in all protease mechanistic classes. In the case of serine (Ser), cysteine (Cys) and threonine (Thr) proteases, the tetrahedral intermediate involves a stable covalent bond to the enzyme’s catalytic nucleophile (Nuc), whereas metalloproteinases and aspartic acid (Asp) proteases use a non-covalent acid–base mechanism. The tetrahedral intermediate is difficult to simulate; protein engineers and chemists have yet to invent or evolve an independent chemical structure or fold that can efficiently catalyse peptide-bond cleavage. There are examples in which catalytic antibodies, for example, have been evolved to efficiently hydrolyse activated esters, but these ester bonds are extremely different from the resonance-stabilized peptide bond124. This is a surprising gap in our knowledge and remains fertile ground for future work.