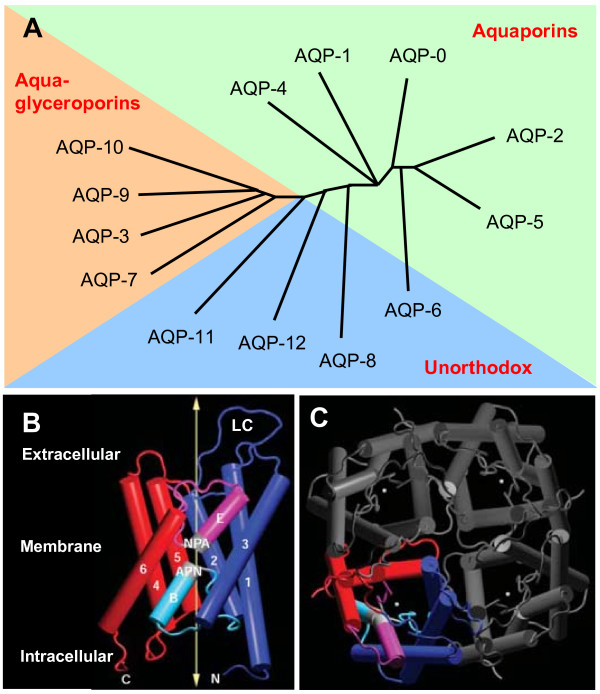
Figure 2.
The aquaporin family tree and the structure of AQP-1. A., This phylogenetic tree shows the relationship of the thirteen water channel proteins in mammals (AQP-0-AQP-12). The assignments in the phylogenetic tree roughly correlate with permeability characteristics. The aquaporins are generally permeated only by water. The aquaglyceroporins are permeated by water and small solutes such as glycerol. The unorthodox were recently named as AQP-11 and AQP-12 were previously named superaquaporins or subcellular AQPs and AQP-6 and AQP-8 were previously named aquaporins. B, The monomeric structure of the aquaporin-1 (AQP-1) is shown with membrane-spanning helices numbered 1-6 displayed as rods and the location of the membrane and the long extracellular C loop (LC). The aminoterminal half of the molecule is shown in purple and light blue, and the carboxy-terminal half is shown in red and pink. Loops B and E, which fold into the membrane to form the pore, are labeled, as are the conserved NPA motifs (shown in light grey). Portions of loops B and E form α-helices, and are therefore shown as rods. The arrow highlights the route taken by water, which can move in both directions through the channel. C, carboxyl terminus; N, amino terminus. C, The AQP-1 tetramer, as seen from above. Asterisks denote the location of the water pore in each subunit. Modified from King after the original published in ref. (15) and reproduced with permission from Nature Publishing Group.