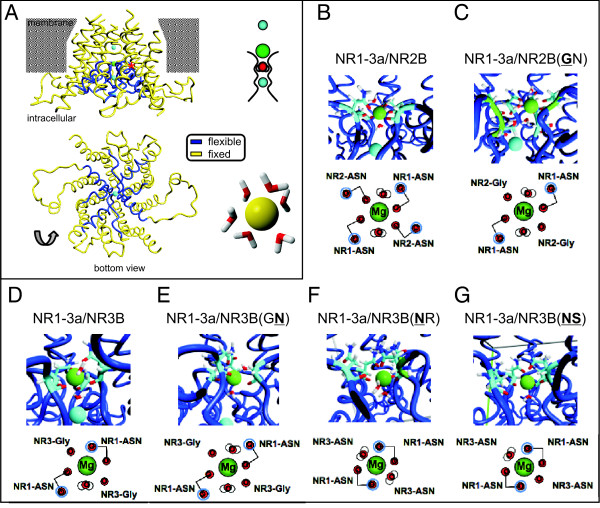
Figure 5.
Coordination of Mg2+ within the pore of wild type and N and N+1 site mutant NMDARs. Conformation of the pore region of an NR1/NR3 diheteromer. A. Side view of the selectivity filter of an NR1/NR3 diheteromer. Note that not the entire transmembrane region is shown. A similar conformation is employed by NR1/NR2 receptors. The N site is located at the tip of the pore loop; the N+1 site is located adjacent to that in the helix (marked by the red asterisk). Below, the same diheteromer is shown in a bottom view. The extensive protein loops belong to the NR1 subunits. To the right, a schematic representation of the selectivity filter of NMDARs is depicted: One Na+ ion (blue ball) and one Mg2+ ion (green ball) are coordinated in the pore with a water molecule (red) serving as a spacer. Bottom right: coordination of six water molecules by an Mg2+ ion. B.-G. Coordination of Mg2+ in NMDAR diheteromers. Mg2+ is depicted as a green ball; the blue ball represents Na+. Below each structure is a schematic view of the coordination by water (red with white protons), side chain oxygens (red dots) and backbone oxygens (red dots with blue rings).