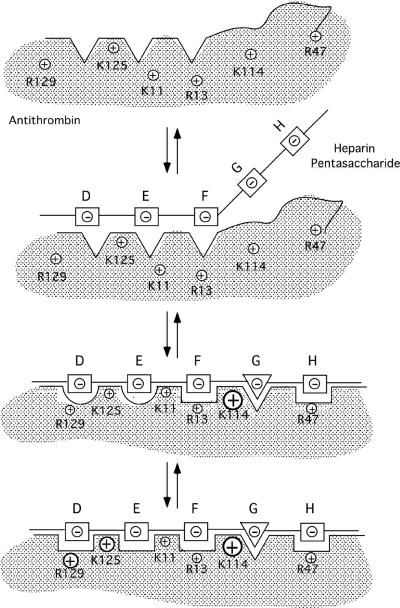
Fig. 9. Induced-fit model for heparin pentasaccharide binding to and allosteric activation of antithrombin.
The pentasaccharide is proposed to bind and allosterically activate antithrombin in 3-steps. ln step one, the conformationally rigid nonreducing end DEF trisaccharide recognizes and binds to the D helix and N-terminal segments of the heparin binding site of antithrombin primarily through electrostatic interactions involving Lys125 and Lys11. The conformationally flexible GH disaccharide, the consequence of an equilibrium between chair and skew boat forms of iduronate residue G, minimally interacts in this step [16]. DEF binding triggers a first set of induced-fit conformational changes in the heparin binding site in step two that include formation of the P helix, rotation of the D helix and bending of the A helix. These changes allow the critical Lys114 interaction with the pentasaccharide 3-O-sulfate to be made and serve to position Lys114 together with Arg13, Arg46, and Arg47 for binding the reducing end GH disaccharide in the skew boat form (triangle). The changes additionally improve the DEF trisaccharide interactions with Lys11, Lys125 and Arg129. A second set of induced-fit conformational changes follows in step three in which the D helix extends at its C-terminal end, causing the buried Tyr131 to become exposed and the hydrophobic core to compact. Such changes increase pentasaccharide complementarity with the heparin binding site by repositioning Lys125 and Arg129 for optimal interactions with the DEF trisaccharide. The relative importance of each basic residue in binding the pentasaccharide in each step is reflected by the size of the positive charge symbols for each residue.