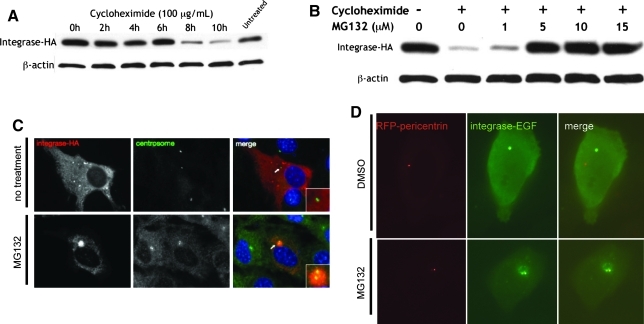
FIG. 6.
ϕC31 integrase protein is degraded within 8 hr by the 26S proteasome. (A) HeLa cells transfected with pCSI-HA were treated with cycloheximide (100 μg/ml) to inhibit protein production and harvested every 2 hr at the indicated times (0–10 hr after adding cycloheximide). Protein levels in the cells were greatly diminished by 8 hr. (B) HeLa cells transfected with pCSI-HA were treated with either no cycloheximide or with cycloheximide at 100 μg/ml (– or+, respectively) and the MG132 concentration as indicated (0, 1, 5, 10, or 15 μg/ml) to inhibit the 26S proteasome. When cells were treated with both inhibitors, the pool of integrase was stabilized. In both (A) and (B), the immunoblot was cut in half at 50 kDa after blocking and incubated with either anti-HA or anti-β-actin primary antibodies. (C) NIH/3T3 cells transfected with pCSI-HA were either untreated (top) or treated with 10 μM MG132 for 10 hr (bottom), methanol-fixed, and stained on coverslips with both anti-HA (integrase-HA, red) and anti-γ-tubulin (centrosome, green) primary antibodies. (D) HeLa cells were cotransfected with pRFP-pericentrin (red) and pPhi-EGFP (green), treated with either 10 μM MG132 (bottom) or the same volume of the solvent DMSO (top) for 8 hr, and live imaged. Only when the 26S proteasome was inhibited could ϕC31 integrase be seen at the centrosome, a site of concentration for the proteasomal machinery. Thus, ϕC31 integrase appeared to be quickly degraded by the 26S proteasome at the centrosome in cultured mammalian cells.