Abstract
Background
Although hip arthroplasty reliably relieves pain and improves function, problems have arisen with wear and osteolysis. Highly crosslinked polyethylene has been developed to address this problem although at present there is limited clinical evidence it does so longer term.
Questions/purposes
We compared the in vivo wear of standard versus highly crosslinked polyethylene (HXLP) in primary total hip arthroplasty at a minimum of 5-year followup.
Methods
We enrolled 122 patients in a prospective, double-blinded, randomized trial and followed them annually to assess their progress. Annual radiographs were analyzed using previously validated edge detection software to assess for two-dimensional, three-dimensional, and volumetric wear. The mean follow up was 5.5 years (range, 4.1 to 7 years).
Results
The two-dimensional wear measurements for HXLP showed lower wear compared to the conventional group (0.05 mm/year versus 0.26 mm/year, respectively). Three-dimensional and volumetric wear were similarly lower in the HXLP group.
Conclusions
Highly crosslinked polyethylene undergoes substantially less wear than conventional polyethylene at medium term. The effect of hip arthroplasty longevity will need to be assessed with longer-term studies, but this may lead to a decreased need for revision as a result of less wear and osteolysis.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA reliably restores function and reduces pain in patients with osteoarthritis [27, 28]. However, there has been much debate as to the optimal bearing coupling with the advent of new hard-on-hard surfaces [15]. There is a potential attraction of reduced wear using a hard bearing couple but this may not be suitable for all patients. Ceramic couples have been noted to squeak [20] or fracture catastrophically [14]. Metal couples have been associated with pseudotumor formation [13], increased metal ion release and potential DNA changes [18]. Metal on polyethylene restores function to patients [5], although these implants are associated with wear and osteolysis [3, 8, 17, 30].
Polyethylene wear debris contributes to osteolysis by activating macrophages to produce osteolytic cytokines [21, 25, 26, 31]. Less wear debris could potentially reduce the amount of osteolysis and improve the longevity of a prosthesis. Highly crosslinked polyethylene is one potential solution to reduce wear [29]. In vitro testing reportedly shows a reduction in wear using both roughened and smooth metal heads articulating against highly crosslinked polyethylene [24].
Marathon polyethylene undergoes 5 MRad irradiation at room temperature followed by remelting for 24 hours at 155°C. Final sterilization occurs via gas plasma in an inert environment. Enduron is not irradiated and undergoes gas plasma sterilization in an inert environment only. The effect of the irradiation is to increase the crosslinking in an attempt to increase its resistance to wear.
We hypothesized HXLP would be associated with less wear than standard polyethylene in primary THA.
Patients and Methods
We designed a prospective, double-blind, randomized trial to compare conventional (Enduron; DePuy, Warsaw, IN) and highly crosslinked (Marathon; DePuy, Warsaw, IN) acetabular liners. Reported wear rates for the conventional crosslinked polyethylene (Enduron) of 0.125 mm/year (SD, 0.089) were used to evaluate the sample size. The manufacturers’ in vitro tests showed an 86% reduction in wear using the highly crosslinked liner (Marathon). Using a more conservative estimate of 50% wear reduction, a reduction of 0.3125 mm wear at 5 years needed to be shown. Assuming a normally distributed primary variable (linear wear at 5 years) with a SD of 0.445, a required level of statistical significance of alpha = 0.05, and a power of 1-beta = 0.95, 55 patients per group would be required to detect a difference of 0.3125 mm at 5 years. We included only patients with age between 45 and 75 years, noninflammatory arthritis, ability to give informed consent, and willingness to participate in the followup protocol. We excluded patients with inflammatory arthritis, pathology requiring nonstandard prostheses, pregnancy, physical or mental disability that could impair a patient’s decision-making capability of giving informed consent, and medical comorbidities that impair the ability to ambulate (Charnley C classification). One hundred twenty-two eligible patients were recruited between June 2001 and September 2003. Sixty-one were randomized into the Marathon group and 61 into the Enduron group. The average age of the patients undergoing surgery was 61 years (range, 46–75 years) with a mean follow up of 5.5 years; range, 4.1–7.0 years. Of the initial 122 patients, four were lost to followup, one had an acetabular revision for recurrent dislocation, and seven had died at the time of review, leaving 110 patients available for assessment (55 in each group). Approval for the study was obtained through the Regional Ethics Committee before beginning the study.
Randomization was by sealed envelopes opened intraoperatively by the circulating nurse to determine the type of polyethylene to be used. The operating surgeon was able to tell the size of the insert from the packaging but not which type of polyethylene was being used. Blinding was maintained for the surgeon, clinical and radiographic assessors, and the patients. The code for the treatment group allocation was kept solely by the research nurse (KA) available only in case of adverse postoperative events and to the statistician for data analysis.
Before entry into the study, all patients underwent anesthetic review and routine preoperative assessment to ensure they fit the inclusion criteria.
All operations were carried out by the two senior surgeons involved in the study (JGH, PD) in a conventional theater. Preoperative antibiotics were administered and the type of anesthetic was decided by the anesthesiologist. Surgery was performed in the lateral position with appropriate supports. The posterior or direct lateral approach was used at the discretion of the surgeon. An uncemented shell (Duraloc Sector; DePuy) with two 6.5-mm cancellous screw augmentation was used having underreamed by 1 mm to achieve an initial press fit. All patients received a Charnley Elite Size 2 roundback (DePuy) cemented femoral stem, a 28-mm cobalt chromium head (Articuleze; DePuy), and a 28-mm inner diameter polyethylene liner.
The postoperative rehabilitation regime included immediate weightbearing and daily supervised physiotherapy as an inpatient. Patients were mobilized on the first postoperative day using a walker and progressing to crutches. Patients were given written and verbal instructions to continue exercises post discharge.
We followed patients at 6 weeks, 6 months, 1 year, and annually up to 6 years at their clinic visit. Patients were monitored for adverse events such as reoperations, dislocations, deep vein thromboses, pulmonary emboli, infection, or death and were reviewed on an annual basis. Radiographs were obtained preoperatively as well as at each clinic visit. Anteroposterior pelvis and crosstable lateral views were ordered routinely. The standard technique for this has been described previously [7].
All radiographs were examined for loosening of the components, wear, and osteolysis. All images were scanned using a Scanmaker 9800XL (Microtek, Singapore) or obtained in high-quality TIF format from the PACS system (Kodak, New York, NY) ensuring a resolution of at least 300 dots per inch. Image analysis was performed using previously validated [8, 16] wear measurement software (Polyware Auto; Draftware Developers Inc, Vevay, IN). Polyware Auto uses automated edge detection techniques that eliminate human error from taking wear measurements manually and increases accuracy threefold and reproducibility 40-fold in comparison to manual techniques [8].
“Bedding-in” or “creep” of the polyethylene is a recognized phenomenon in the first 6 months [12] and it is important not to use radiographs in this period as a result of the error they may infer. To reduce the disproportionate effects of bedding-in and creep, the initial radiograph used was that taken 6 months after surgery. Linear, three-dimensional and volumetric wear were measured using the software that automatically accounts for the varying projections of different radiographs. The lateral radiograph is used to measure out-of-plane wear enabling three-dimensional and volumetric wear to be measured as well as linear wear.
We determined difference in age, gender, surgeon, and cup position using the chi square test for ratios and the Mann-Whitney U test for continuous data. We observed no differences between groups with respect to age, gender, operative side, surgeon, cup size, or cup positioning (anteversion or abduction angle) (Table 1).
Table 1.
Demographic, clinical and radiological data
| Patient demographics and implant data | Enduron Median (range) or ratio | Marathon Median (range) or ratio |
|---|---|---|
| Age | 61 (48–75) | 62 (46–75) |
| Male:female | 26:29 | 35:20 |
| Left:right ratio | 23:32 | 25:30 |
| Surgeon (Horne-Devane) | 37:18 | 29:26 |
| Cup size | 54 (48–58) | 54 (48–60) |
| Abduction angle | 43.4 (28.2–55.2) | 40.9 (23.7–58.6) |
| Anteversion | 11.5 (−11.9–32.6) | 8.2 (−3.8–31) |
Differences in linear, three-dimensional, and volumetric wear between the two groups was determined with a statistical software package (SAS, Version 9.1; SAS Institute Inc, Cary, NC) using the Proc Mixed procedure. Parameters were fit to estimate the mean difference in wear between the liner types, the linear slope of wear across time, and an interaction term to explicitly test whether the slope of wear over time was substantially different between the two liner types. Followup time was entered into the equation as a continuous variable, because followup radiographs did not always exactly coincide with the annual scheduling.
Results
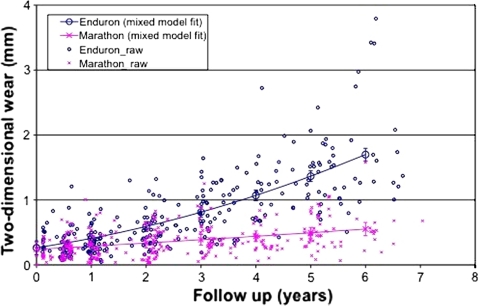
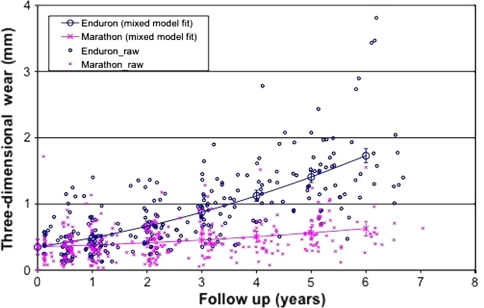
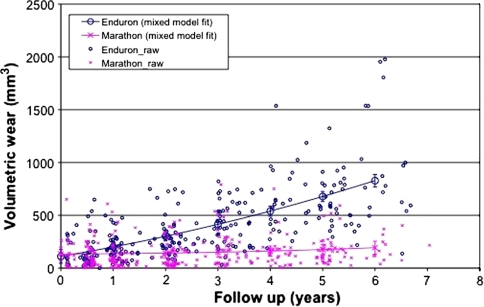
We observed reduced (p < 0.001) two-dimensional wear in the highly crosslinked polyethylene wear group compared with the standard crosslinked polyethylene group. This became significant at 2 years and thereafter (Fig. 1). The wear measurement software uses the lateral radiograph to calculate three-dimensional wear which was also reduced (p < 0.001) in the highly crosslinked group (Fig. 2). The effect of this is to reduce volumetric wear (Fig. 3). The two-dimensional wear rate for the highly crosslinked polyethylene was less (p < 0.001) than that for the standard crosslinked polyethylene: 0.05 mm per year (95% CI = 0.03-0.07 versus 0.26 mm per year (95% confidence interval = 0.23 to 0.28). The wear rates were similar for three-dimensional and volumetric wear (Table 2). After adjusting for length of followup, standard crosslinked polyethylene had more (p < 0.0001) two-dimensional wear than the highly crosslinked polyethylene (difference = 0.96 mm, 95% CI = 0.59–1.32). There was also more (p < 0.001) three-dimensional wear (difference = 0.93 mm, 95% CI = 0.57–1.29) and volumetric wear (difference = 4.39 mm3, 95% CI = 3.04–6.31).
Fig. 1.
The graph shows reduced two-dimensional wear with time of highly versus standard crosslinked polyethylene.
Fig. 2.
The graph shows reduced three-dimensional wear with time of highly versus standard crosslinked polyethylene.
Fig. 3.
Volumetric wear with time (standard versus highly crosslinked polyethylene) is shown in this graph.
Table 2.
Wear for standard versus highly crosslinked polyethylene liners
| Type of polyethylene | 2D Wear (mm/yr) | 3D wear (mm/yr) | Vol wear (mm3/yr) | |||
|---|---|---|---|---|---|---|
| Creep | Wear | Creep | Wear | Creep | Wear | |
| Standard cross linked polyethylene (95% CI) | 0.37 | 0.26 (0.23–0.28) | 0.46 | 0.24 (0.21–0.27) | 195.28 | 123.47 (109.60–137.34) |
| Highly cross linked polyethylene (95% CI) | 0.30 | 0.05 (0.03–0.07) | 0.39 | 0.04 (0.02–0.07) | 134.83 | 10.65 (−3.65–24.94) |
Postoperative complications (Table 3) included one pulmonary embolus secondary to a deep vein thrombosis and one patient had a deep infection. There were seven dislocations treated with bracing after closed reduction. There has been one revision for instability as a result of polyethylene wear (standard polyethylene group) and there are two patients awaiting revision surgery for aseptic loosening of the stem.
Table 3.
List of complications
| Complications | Number (%) |
|---|---|
| DVT/PE | 1 (0.8) |
| Dislocations | 7 (5.9) (4 HXLP patients, 3 standard poly) |
| Heterotopic ossification | 1 (0.8) |
| Infection | 1 (0.8) |
| Revision | 1 (0.8) |
| Awaiting revision | 2 (1.6) (Both standard poly) |
Discussion
Total hip arthroplasty reliably relieves pain and improves function but longer term durability is limited by wear and osteolysis. HXLP was developed to reduce wear and osteolysis. One in vitro study [24] suggests it is more resistant to wear, but limited longer term clinical evidence is available to document whether it does [1, 6, 10, 11]. To confirm these studies we asked whether using HXLP would reduce the wear of polyethylene in primary total hip arthroplasty. HXLP is a potentially attractive bearing surface if the rates of wear and associated osteolysis can be reduced as compared to standard crosslinked polyethylene.
Despite our findings, the study has several limitations. First, there are various methods of producing HXLP, including production, annealing, and irradiation, which may influence wear properties. Some concern has been expressed as to the brittleness and resistance to fatigue of HXLP. Currier et al. [4] analyzed retrieved acetabular liners showing oxidation and fatigue damage although they had not failed clinically and had been explanted for other reasons. Thus, our data apply only to the particular product we studied. Second, these are only midterm results. Despite showing reduced wear, longer term results (10 years and beyond) are important to show whether the use of HXLP improves longevity of total hip arthroplasty. There will be less production of polyethylene debris, but these midterm results cannot exclude osteolysis at a later date. Third, this study was designed to look at wear and not specifically at osteolysis. Other studies using radiographs and computed tomography have identified less osteolysis using highly crosslinked polyethylene [2, 19]. A threshold of 0.2 mm/year of wear has been postulated as the level at which osteolysis may occur [22]. The Enduron polyethylene is above this but the Marathon HXLP is substantially below it. The strengths of this study include the randomization, blinding, standardization of implants, and the high followup ratio giving the results statistical validity. This study does not attempt to correlate wear with clinical results, although that could be assessed with the ongoing 10-year analysis in the future.
Our data suggest highly crosslinked polyethylene (HXLP) is associated with less wear over time compared with standard crosslinked polyethylene patients. This supports the in vitro testing of similar types of polyethylene [24].The lower wear rates we found are consistent with those in other studies showing a reduction in wear at 5 years. Engh et al. [11] and Bitsch et al. [1] showed reduced wear with polyethylene from the same manufacturer as the HXLP in our study and other authors have reported similar findings with HXLP from other manufacturers including Crossfire by Stryker (Kalamazoo, Michigan) [6], and Durasul from Zimmer (Warsaw, Indiana) [10]. The study of Engh et al. [11] differed from ours because it used lateralized liners and two different types of femoral stem. The paper of Bitsch et al. [1] was based on a smaller cohort of 58 patients and used a different but validated wear measurement software. It is difficult to compare these studies due to the variables in HXLP production between companies, methodology, accounting for bedding in and the type of wear measurement software utilized. It is, however, of note that all these studies do show reduced wear with HXLP at early and mid term results.
Recognition of initial creep or “bedding-in” of components is vital to produce accurate results of wear. Ninety-five percent of head penetration in the first 6 months has been attributed to creep [12], which must be separated from wear. In this series, the first reference radiograph was taken 6 months postoperatively to account for creep, which would otherwise have had a disproportionate effect on the wear rates recorded. There was 0.30 mm of two dimensional wear in the first 6 months for HXLP compared with an annual wear of 0.05 mm showing the effect of this initial bedding in (Table 3). Not all previous studies have allowed for this [9, 32]. Similar amounts of bedding in were noted for both types of polyethylene (Table 3).
It took 2 years in this study for the differential wear rates to become significant. This contrasts with a recent study by McCalden et al., which took 5 years to detect a significant difference [23]. Their study had several differences, including a smaller cohort of patients (100), use of a different type of validated wear measurement software, and a different method of statistical analysis.
In conclusion, this study confirms previous findings of decreased wear in highly crosslinked polyethylene liners compared with standard polyethylene. The effect of hip arthroplasty longevity will need to be assessed with longer-term studies, but this may lead to a decreased need for revision.
Acknowledgments
We thank James Stanley, Department of Public Health, Wellington School of Medicine (Wellington, New Zealand), for his assistance with statistical analysis of the data.
Footnotes
One of the authors (PAD) certifies that he has or may receive payments or benefits from a commercial entity (DePuy International) related to consultancy work. The institution of the authors (PAD, JGH, KA) has received funding from DePuy International and the Wellington Surgical Research Trust.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Wellington and Wakefield Hospitals, Wellington, New Zealand.
References
- 1.Bitsch RG, Loidolt T, Heisel C, Ball S, Schmalzried TP. Reduction of osteolysis with use of Marathon cross-linked polyethylene. A concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2008;90:1487–1491. doi: 10.2106/JBJS.F.00991. [DOI] [PubMed] [Google Scholar]
- 2.Bragdon CR, Kwon YM, Geller JA, Greene ME, Freiberg AA, Harris WH, Malchau H. Minimum 6-year followup of highly cross-linked polyethylene in THA. Clin Orthop Relat Res. 2007;465:122–127. doi: 10.1097/BLO.0b013e31815760b1. [DOI] [PubMed] [Google Scholar]
- 3.Cooper RA, McAllister CM, Borden LS, Bauer TW. Polyethylene debris-induced osteolysis and loosening in uncemented total hip arthroplasty. A cause of late failure. J Arthroplasty. 1992;7:285–290. doi: 10.1016/0883-5403(92)90050-Z. [DOI] [PubMed] [Google Scholar]
- 4.Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Citters DW. Evaluation of oxidation and fatigue damage of retrieved crossfire polyethylene acetabular cups. J Bone Joint Am. 2007;89:2023–2029. doi: 10.2106/JBJS.F.00336. [DOI] [PubMed] [Google Scholar]
- 5.Cushnaghan J, Coggon D, Reading I, Croft P, Byng P, Cox K, Dieppe P, Cooper C. Long-term outcome following total hip arthroplasty: a controlled longitudinal study. Arthritis Rheum. 2007;57:1375–1380. doi: 10.1002/art.23101. [DOI] [PubMed] [Google Scholar]
- 6.D’Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Devane PA, Bourne RB, Rorabeck CH, MacDonald S, Robinson EJ. Measurement of polyethylene wear in metal-backed acetabular cups. II. Clinical application. Clin Orthop Relat Res. 1995;319:317–326. [PubMed] [Google Scholar]
- 8.Devane PA, Horne JG. Assessment of polyethylene wear in total hip replacement. Clin Orthop Relat Res. 1999;369:59–72. doi: 10.1097/00003086-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Digas G, Kärrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. doi: 10.1097/01.blo.0000150314.70919.e3. [DOI] [PubMed] [Google Scholar]
- 10.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 11.Engh CA, Jr, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Jr, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21(Suppl 2):17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90:556–561. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- 13.Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br. 2009;91:1566–1574. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 14.Ha YC, Kim SY, Kim HJ, Yoo JJ, Koo KH. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin Orthop Relat Res. 2007;458:106–110. doi: 10.1097/BLO.0b013e3180303e87. [DOI] [PubMed] [Google Scholar]
- 15.Heisel C, Silva M, Schmalzried TP. Bearing surface options for total hip replacement in young patients. Instr Course Lect. 2004;53:49–65. [PubMed] [Google Scholar]
- 16.Hui AJ, McCalden RW, Martell JM, MacDonald SJ, Bourne RB, Rorabeck CH. Validation of two and three-dimensional radiographic techniques for measuring polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:505–511. doi: 10.2106/00004623-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh BF, Dewitz MA, Ilstrup DM, Stauffer RN, Coventry MB. Charnley total hip arthroplasty with cement. Fifteen-year results. J Bone and Joint Surg Am. 1989;71:1496–1503. [PubMed] [Google Scholar]
- 18.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):78–83. [DOI] [PubMed]
- 19.Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA, Jr, Engh CA., Sr Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty. 2007;22(Suppl 2):134–139. doi: 10.1016/j.arth.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Mai K, Verioti C, Ezzet KA, Copp SN, Walker RH, Colwell CW., Jr Incidence of ‘squeaking’ after ceramic-on-ceramic total hip arthroplasty. Clin Orthop Relat Res. 2010;468:413–417. doi: 10.1007/s11999-009-1083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloney WJ, Galante JO, Anderson M, Goldberg V, Harris WH, Jacobs J, Kraay M, Lachiewicz P, Rubash HE, Schutzer S, Woolson ST. Fixation, polyethylene wear, and pelvic osteolysis in primary total hip replacement. Clin Orthop Relat Res. 1999;369:157–164. doi: 10.1097/00003086-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20:880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 23.McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 24.McKellop H, Shen FW, DiMaio W, Lancaster JG. Wear of gamma-crosslinked polyethylene acetabular cups against roughened femoral balls. Clin Orthop Relat Res. 1999;369:73–82. doi: 10.1097/00003086-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72:22–28. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- 26.Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85:1095–1099. doi: 10.2106/00004623-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Rissanen P, Aro S, Slätis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10:169–175. doi: 10.1016/S0883-5403(05)80123-8. [DOI] [PubMed] [Google Scholar]
- 28.Ritter MA, Albohm MJ, Keating EM, Faris PM, Meding JB. Comparative outcomes of total joint arthroplasty. J Arthroplasty. 1995;10:737–741. doi: 10.1016/S0883-5403(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 29.Santavirta S, Böhler M, Harris WH, Konttinen YT, Lappalainen R, Muratoglu O, Rieker C, Salzer M. Alternative materials to improve total hip replacement tribology. Acta Orthop Scand. 2003;74:380–388. doi: 10.1080/00016470310017668. [DOI] [PubMed] [Google Scholar]
- 30.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 31.Sochart DH. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop Relat Res. 1999;363:135–150. doi: 10.1097/00003086-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Triclot P, Grosjean G, El Masri F, Courpied JP, Hamadouche M. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking. A prospective randomised trial. J Bone Joint Surg Br. 2007;89:1439–1445. doi: 10.1302/0301-620X.89B11.19543. [DOI] [PubMed] [Google Scholar]