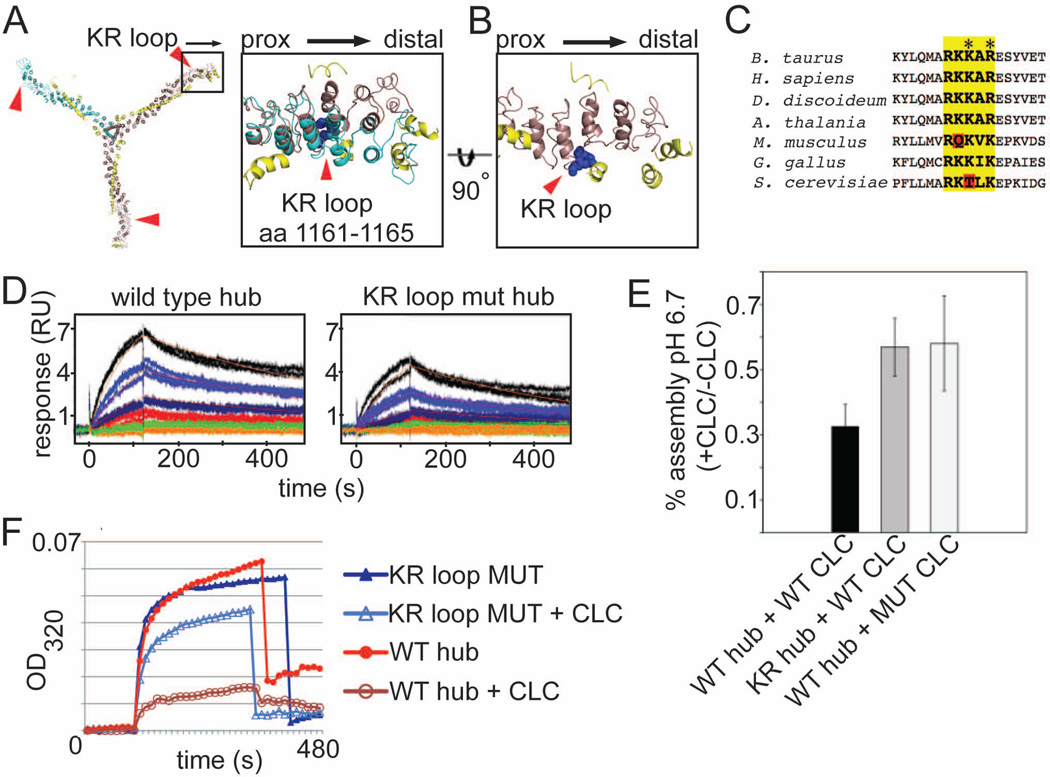
Figure 2. The KR loop in the clathrin heavy chain knee participates in electrostatic regulation of assembly by clathrin light chain.
(A) Expanded boxed region shows the position of the KR loop (blue spheres, residues 1161–1165 in CHC) relative to CLC. The straight knee conformation (brown) associated with the extended form of CLC (yellow) and the bent knee conformation (cyan) associated with the compact form of CLC (not shown) are overlaid. The KR loop is indicated by red arrowheads. Major deviations between the overlaid structures occur distal to the KR loop. (B) Expanded box region of a, rotated 90° (straight knee only). (C) Alignment of various CHC protein sequences around the KR loop (bold, yellow highlight). Red highlights show differences that alter the charge distribution. Residues mutated to alter the KR loop charge indicated by asterisks (K1163E and R1165D). (D) Surface plasmon resonance (SPR) analysis of clathrin light chain interactions with wild type clathrin hub or KR loop mutant (mut) hub. Human neuronal LCb was immobilized on the SPR chip surface and wild type or KR loop mutant hub flowed over at different concentrations. The fitted binding affinity for wild type was 25 nM while KR loop mutant was 59.1 nM. The difference in affinity for CLC was due to a slowed on-rate for the KR loop mutant likely due to decreased electrostatic attraction. (E) Purified hubs (wild-type, WT or mutant, KR) were saturated with CLC (wild type neuronal LCb (WT CLC) or neuronal LCb with E20ED22 mutated to K20KK22 (MUT CLC)) or left unoccupied and assayed for assembly at pH 6.7 by light scattering. The ratio of assembly signal for hub with light chain to assembly signal for hub without light chain for is plotted for each reaction (mean±SEM). (F) Representative kinetic assembly data for WT or KR loop mutant (MUT) hub with or without CLC bound, as indicated. Assembly is detected photometrically by an increase in light scatter at OD320.