Abstract
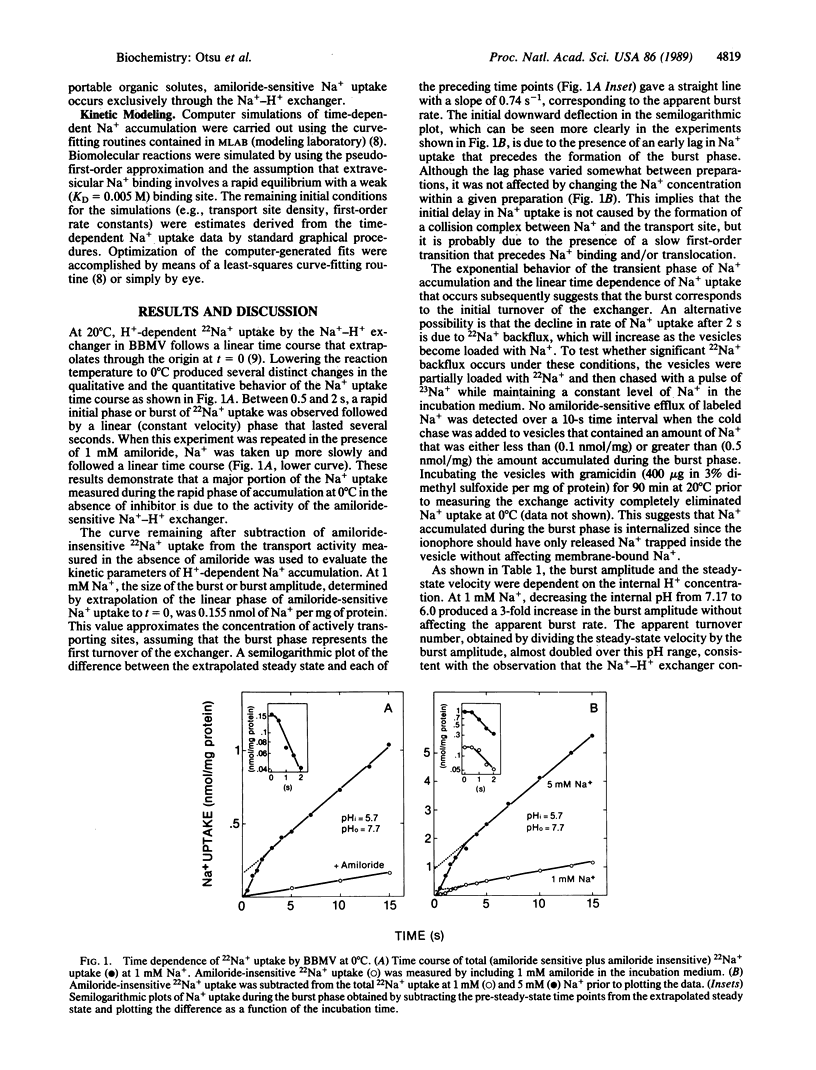
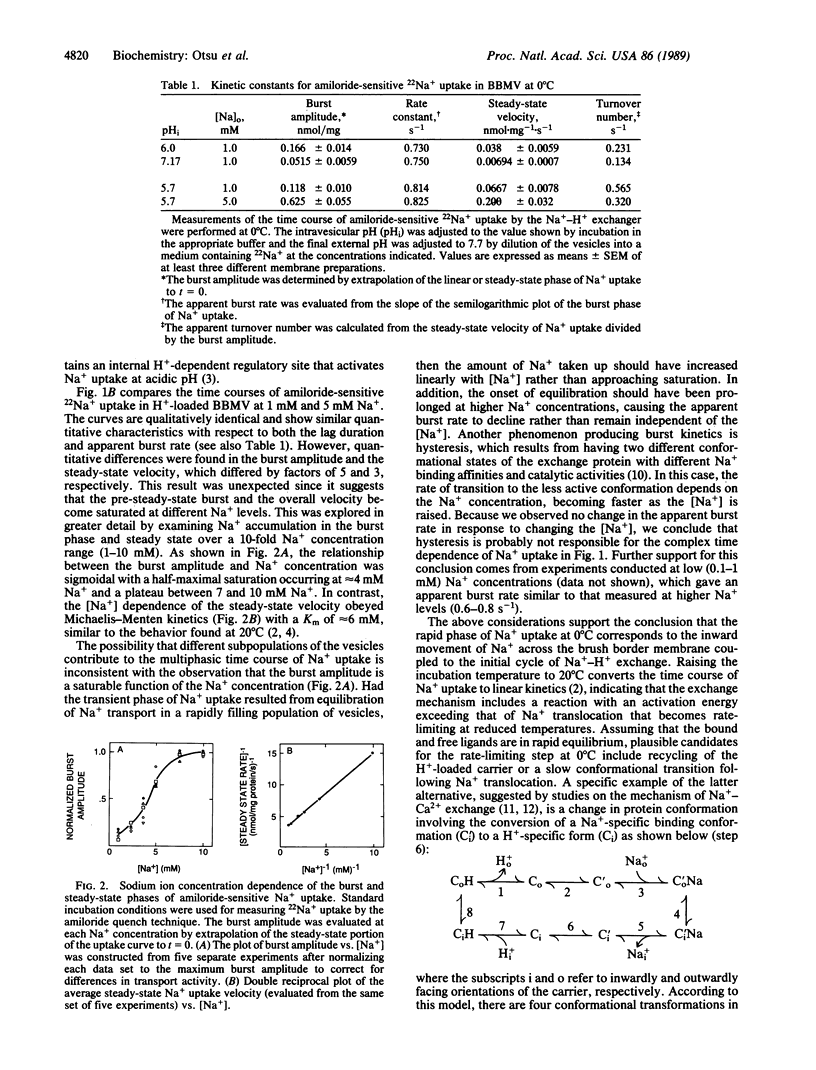
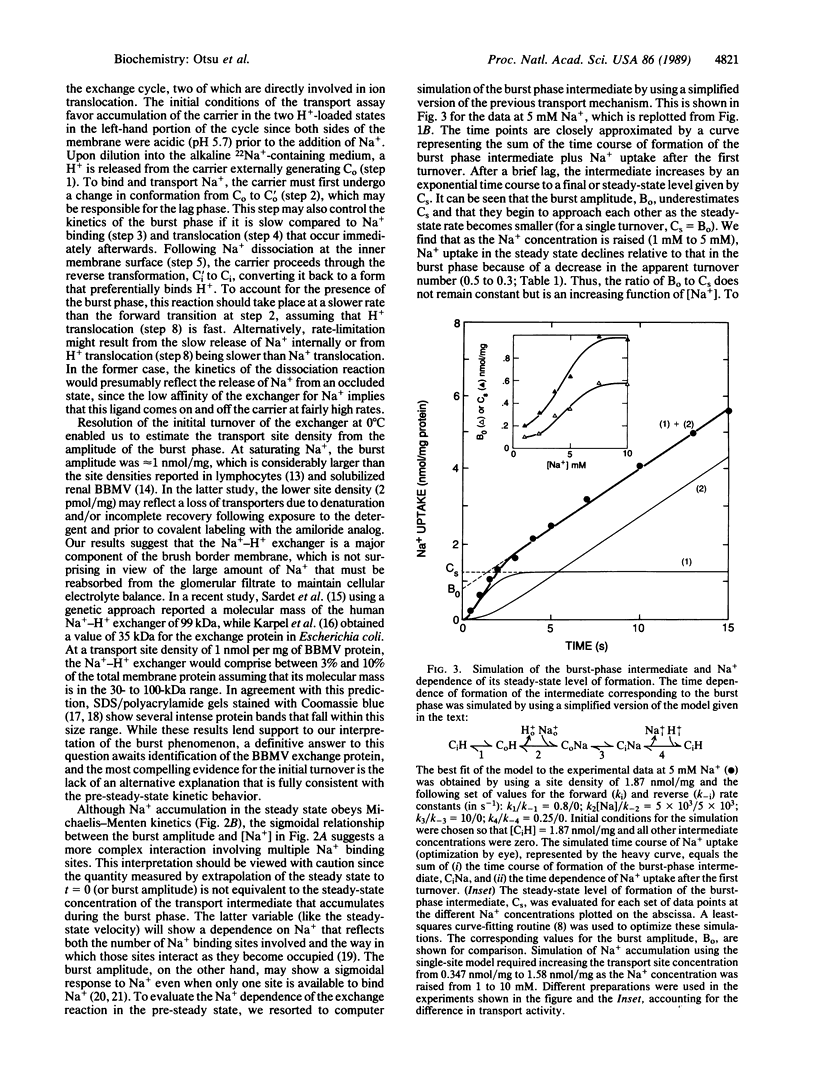
Pre-steady-state kinetic measurements of 22Na+ uptake by the amiloride-sensitive Na+-H+ exchanger in renal brush border membrane vesicles (BBMV) were performed at 0 degrees C to characterize the intermediate reactions of the exchange cycle. At 1 mM Na+, the initial time course of Na+ uptake was resolved into three separate components: (i) a lag phase, (ii) an exponential or "burst" phase, and (iii) a constant velocity or steady-state phase. Pulse-chase experiments using partially loaded BBMV showed no evidence for 22Na+ back-flux, suggesting that the decline in the rate of Na+ uptake rate following the burst represents completion of the first turnover of the exchanger. Gramicidin completely abolished Na+ uptake, indicating that the burst phase results from the translocation of Na+ rather than from residual Na+ binding to external sites. Raising the [Na+] from 1 to 10 mM at constant pH (internal pH 5.7; external pH 7.7) produced a sigmoidal increase in the amplitude of the burst phase without affecting the lag duration or the apparent burst rate. In contrast, Na+ uptake in the steady state obeyed Michaelis-Menten kinetics. These results suggest that a minimum of two Na+ transport sites must be occupied to activate Na+ uptake in the pre-steady state. The transition to Michaelis-Menten kinetics in the steady state can be explained by a "flip-flop" or alternating site mechanism in which the functional transport unit is an oligomer and only one promoter per cycle is allowed to form a translocation complex with Na+ after the first turnover.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Chappelet-Tordo D., Fosset M., Iwatsubo M., Gache C., Lazdunski M. Intestinal alkaline phosphatase. Catalytic properties and half of the sites reactivity. Biochemistry. 1974 Apr 23;13(9):1788–1795. doi: 10.1021/bi00706a002. [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Cohen S., Cragoe E. J., Jr, Grinstein S. Estimation of the number and turnover rate of Na+/H+ exchangers in lymphocytes. Effect of phorbol ester and osmotic shrinking. J Biol Chem. 1987 Mar 15;262(8):3626–3632. [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Hansen V. A., Morrissey J. J. ADP ribosylation of canine renal brush border membrane vesicle proteins is associated with decreased phosphate transport. J Biol Chem. 1982 Oct 25;257(20):12380–12386. [PubMed] [Google Scholar]
- Karpel R., Olami Y., Taglicht D., Schuldiner S., Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10408–10414. [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange in isolated renal brush-border membrane vesicles in response to metabolic acidosis. Kinetic effects. J Biol Chem. 1984 Nov 10;259(21):13224–13227. [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Competitive interactions of sodium and calcium with the sodium-calcium exchange system of cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3178–3182. [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Vigne P., Jean T., Barbry P., Frelin C., Fine L. G., Lazdunski M. [3H]Ethylpropylamiloride, a ligand to analyze the properties of the Na+/H+ exchange system in the membranes of normal and hypertrophied kidneys. J Biol Chem. 1985 Nov 15;260(26):14120–14125. [PubMed] [Google Scholar]
- Weinman E. J., Shenolikar S., Kahn A. M. cAMP-associated inhibition of Na+-H+ exchanger in rabbit kidney brush-border membranes. Am J Physiol. 1987 Jan;252(1 Pt 2):F19–F25. doi: 10.1152/ajprenal.1987.252.1.F19. [DOI] [PubMed] [Google Scholar]


