Figure 2.
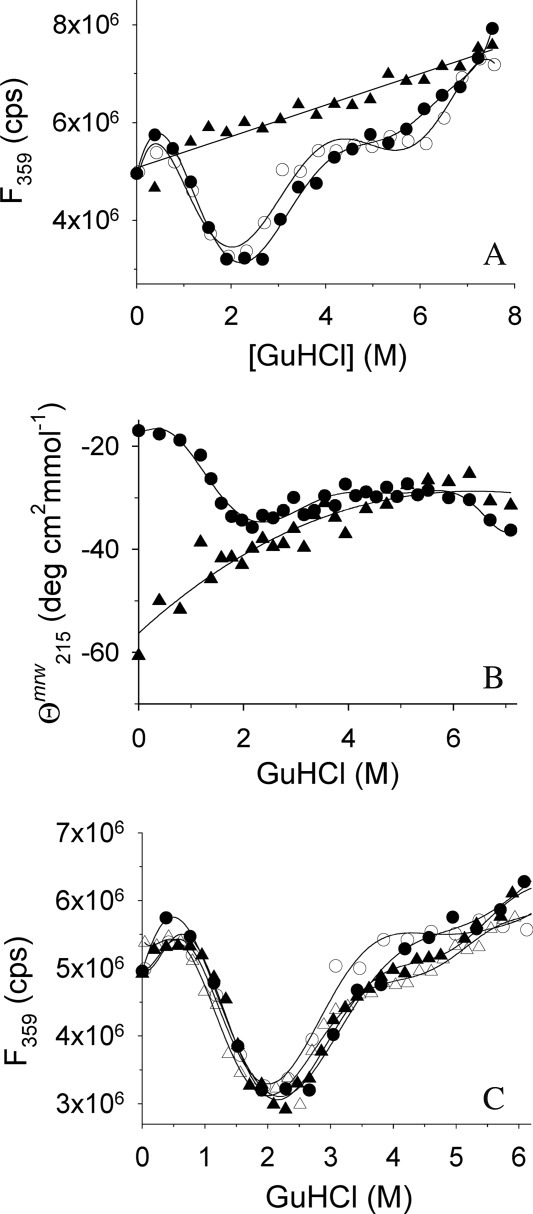
GuHCl-induced unfolding of rPA and IAA-rPA. (A) GuHCl-induced unfolding and refolding of rPA and IAA-rPA, respectively, were monitored by tryptophan fluorescence emission at 359 nm. Excitation wavelength was 280 nm. Data points represent the unfolding of rPA (filled circles), the refolding of rPA (open circles), and the unfolding of IAA-rPA (filled triangles). The protein concentration was 10 μg mL−1. (B) GuHCl-induced unfolding of rPA (filled circles) and IAA-rPA (filled triangles), respectively, were monitored by far-UV CD at 215 nm in the presence of 100 mM d,l-ArgHCl. The protein concentration for rPA was 440 μg mL−1 rPA and 15 μg mL−1–193 μg mL−1 for IAA-rPA. (C) The influence of l-ArgHCl on the GuHCl-induced unfolding and refolding of rPA was monitored by tryptophan fluorescence. In addition to the data from (A), data points are shown for the unfolding (filled triangles) and the refolding of rPA (open triangles) in the presence of 200 mM l-ArgHCl. Lines are meant to guide the eye.
