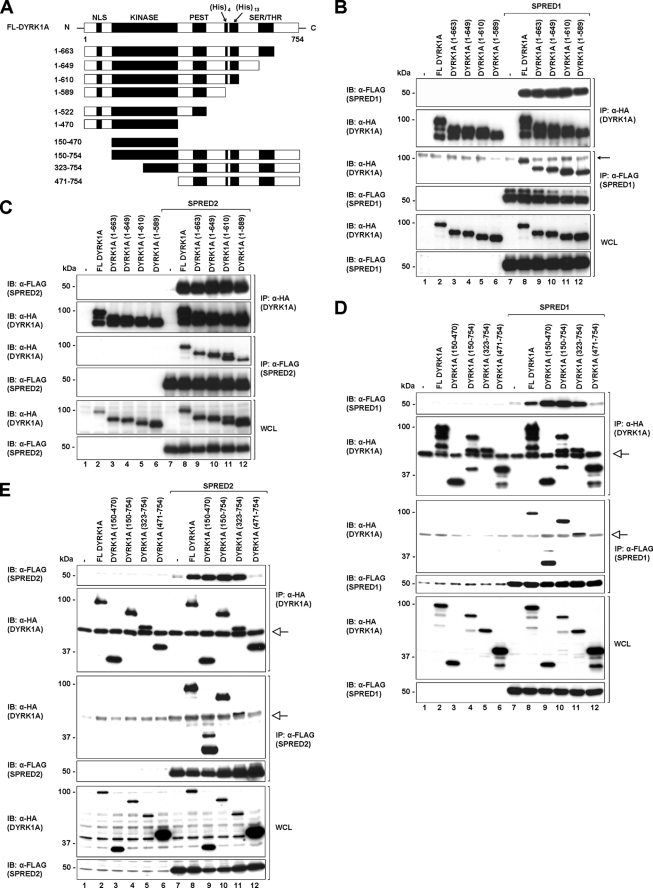
FIGURE 2.
SPRED1 and -2 interact with the kinase domain of DYRK1A. A, schematic diagram showing full-length and different truncation constructs of DYRK1A that were used in the subsequent studies. B and C, full-length, C-terminal truncated mutants (1–663, 1–649, 1–610, and 1–589) of DYRK1A and full-length SPRED1 (B) and SPRED2 (C) were transfected in 293 cells. Cell lysates were immunoprecipitated (IP) with FLAG or rat HA antibodies. Immunoprecipitates and WCL were resolved by SDS-PAGE and immunoblotted (IB) with the antibodies indicated on the left. Arrow indicates a nonspecific band. The band of the FL DYRK1A protein in the anti-FLAG complex seen in lane 8 (B, 3rd panel) is higher in intensity compared with the nonspecific band. D and E, 293 cells were co-transfected with full-length DYRK1A, the kinase domain of DYRK1A(150–470), and three N-terminal truncates of DYRK1A (150–754, 323–754, and 471–754) as indicated. Cell lysates were immunoprecipitated with anti-FLAG and rat anti-HA, and the precipitated proteins were separated on SDS-PAGE and analyzed by Western blotting techniques with the indicated antibodies. Open arrow indicates the immunoglobulin heavy chain.