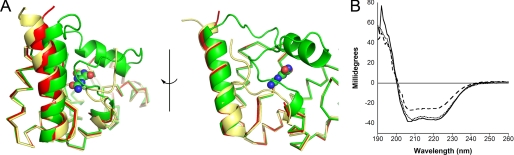
FIGURE 4.
Conformational change of KpOHCU decarboxylase. A, ribbon diagrams show the structural superposition of KpOHCU decarboxylase-allantoin complex (green), unliganded KpOHCU decarboxylase in tetragonal form (yellow), and KpOHCU decarboxylase treated with allopurinol (red). The r.m.s.d. for the superposition of the allantoin-bound structure to the unliganded is 0.499, whereas the r.m.s.d. for the allopurinol-soaked structure to the unliganded is 0.346. Allantoin is shown in space-fill representation. Helix 6 (above allantoin) is visible only in the allantoin bound structure. B, circular dichroism measurements are shown of unliganded KpOHCU decarboxylase (dotted line), KpOHCU decarboxylase treated with allantoin (solid line), and KpOHCU decarboxylase treated with allopurinol (dashed line). The increase in signal at 208 and 222 nm and the decrease in signal at 193 nm in the presence of allopurinol are all consistent with a loss of helical content.