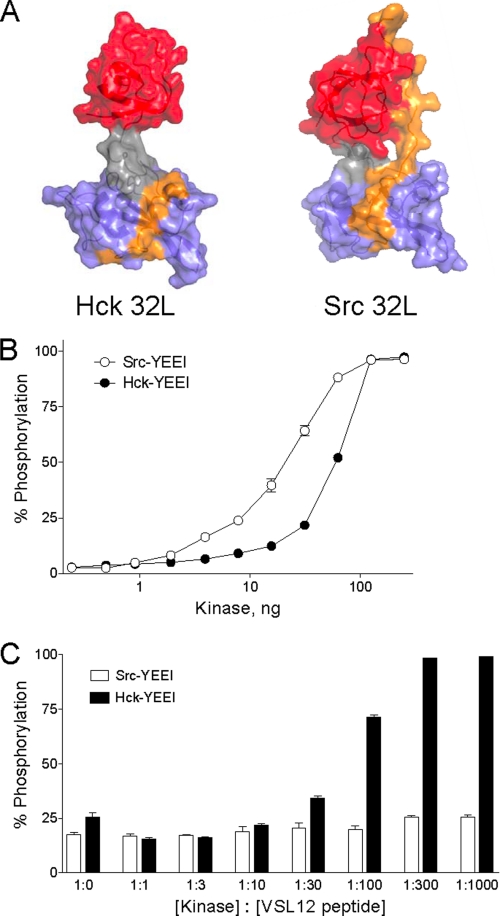
FIGURE 6.
Activation of Hck but not c-Src by an SH3-binding peptide. A, comparison of the Hck32L crystal structure (this study) and the corresponding SH3-SH2 linker region from an active c-Src structure (PDB code 1Y57). In the c-Src structure, the SH3-SH2 linker region is uncoupled from the kinase domain (see text for details). The SH3 domain is rendered in red; the SH3-SH2 connector in gray; the SH2 domain in blue, and the linker in orange. Note that the linker remains bound to the SH3 domain in the c-Src structure but is disordered and therefore not observed in the Hck32L structure with the exception of SH2 contacts. B, Src-YEEI and Hck-YEEI are active in vitro. Recombinant purified Src-YEEI and Hck-YEEI were titrated into the Z'-LyteTM assay as shown. Each reaction was monitored in triplicate, and data are expressed as percent of peptide phosphorylation relative to a control phosphopeptide ± S.D. C, SH3-binding peptide VSL12 activates Hck-YEEI but not Src-YEEI. In vitro kinase assays were performed using equivalent amounts of Src-YEEI and Hck-YEEI that gave ∼20–25% maximal activity in the absence of the VSL12 peptide. Reactions were run in the presence of increasing kinase: VSL12 peptide ratios as shown. Each reaction was monitored in triplicate, and data are expressed as percent of peptide phosphorylation relative to a control phosphopeptide ± S.D.