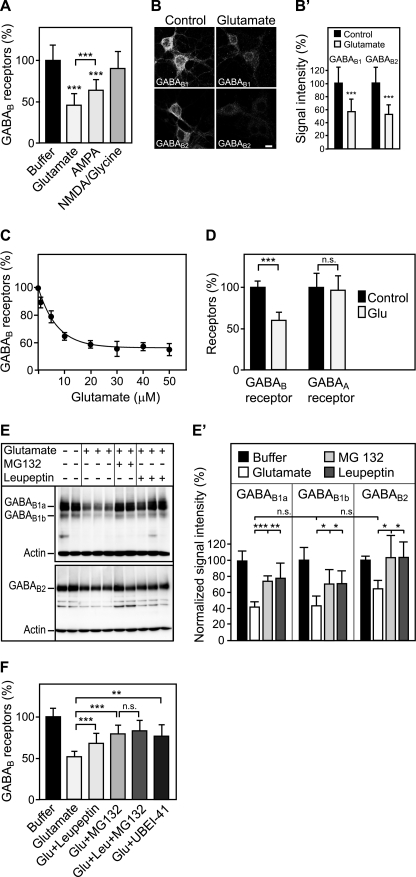
FIGURE 1.
Sustained activation of glutamate receptors induces the down-regulation of GABAB receptors via lysosomal degradation. A, down-regulation of GABAB receptors is triggered by glutamate and AMPA. Primary cortical neurons were treated either with 50 μm glutamate, 100 μm AMPA, or 100 μm NMDA + 10 μm glycine for 90 min and tested for GABAB receptor levels using the in-cell Western assay and antibodies directed against the C terminus of GABAB1. Means ± S.D.; n = 20–40 cultures from three to five preparations; ***, p < 0.001, one-way ANOVA; Bonferoni post test. B, glutamate down-regulates GABAB1 and GABAB2 as verified by immunocytochemistry. For immunofluorescence analysis, cells were treated with 50 μm glutamate for 30 min. This incubation time resulted in sufficient remaining fluorescence signals in the glutamate-treated cultures enabling a reliable quantification. After fixation and permeabilization, neurons were stained with antibodies directed against GABAB1 and GABAB2, respectively. Representative confocal images are shown. B′, quantification of fluorescence signals. Scale bar represents 10 μm. Means ± S.D.; n = 33–48 neurons from three preparations; ***, p < 0.0001, t test. C, dose dependence of glutamate-induced down-regulation of GABAB receptors. Neurons were treated with increasing concentrations of glutamate for 90 min and analyzed for GABAB receptor levels using the in-cell Western assay and GABAB1 antibodies. A concentration of 5 μm glutamate led to half-maximal down-regulation of GABAB receptors. Means ± S.D.; n = 20 cultures from three preparations. D, glutamate does not affect expression levels of GABAA receptors. Neurons were subjected to 50 μm glutamate for 90 min and analyzed for GABAB and GABAA receptor levels using the in-cell Western assay and antibodies directed against GABAB1 and the GABAA receptor α1 subunit, respectively. Means ± S.D.; n = 40–60 cultures from five preparations; ***, p < 0.0001, n.s., p = 0.8, t test. E, glutamate-induced down-regulation is prevented by lysosome and proteasome inhibitors. Neurons were treated with 50 μm glutamate in the presence or absence of the lysosome blocker leupeptin (100 μm) or the proteasome blocker MG132 (10 μm), solubilized and analyzed for GABAB receptor levels by Western blotting using GABAB1 and GABAB2 antibodies. GABAB1a and GABA1b isoforms as well as GABAB2 subunits were down-regulated after glutamate treatment. Down-regulation was blocked by the lysosome inhibitor leupeptin and by the proteasome inhibitor MG132. Bands present in the middle of the blot were observed at varying intensities and most likely represent degradation products of GABAB receptors. The experiment shown was repeated once with three cultures for each condition. E′, quantification of the Western blots. Means ± S.D.; n = 4–6 cultures; n.s. = p > 0.05, *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA, Dunnett's post test. F, proteasome is most likely not directly involved in down-regulating GABAB receptors. Neurons were treated for 90 min with 50 μm glutamate in the presence or absence of the lysosome blocker leupeptin (100 μm), the proteasome blocker MG132 (10 μm), or a combination of both. In addition, the involvement of ubiquitination was tested by treating neurons with the ubiquitin-activating enzyme (E1) inhibitor UBEI-45 (50 μm). GABAB receptor levels were determined using the in-cell Western assay and GABAB1 antibodies. Because the effects of leupeptin and MG132 did not add up, it is unlikely that GABAB receptors are degraded by proteasomes. Means ± S.D.; n = 40–48 cultures from four preparations; n.s. = p > 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA; Bonferoni post test.