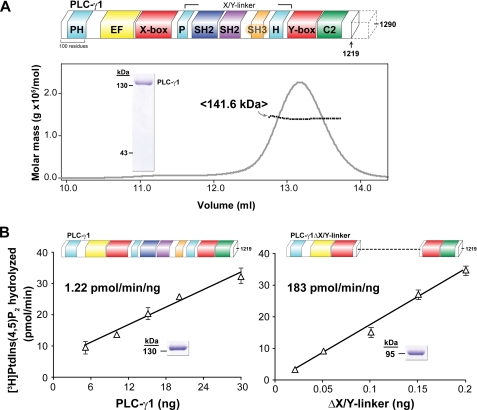
FIGURE 1.
Purified PLC-γ1 is monomeric and auto-inhibited by its X/Y linker. A, purified PLC-γ1 is monomeric. Schematic representation of full-length rat PLC-γ1 (upper panel). PLC isozymes contain a conserved core consisting of an N-terminal PH domain (cyan), an array of four EF hands (yellow), a catalytic TIM barrel composed of X and Y boxes (red), and a C-terminal C2 domain (green). PLC-γ isozymes are unique in containing multiple domains within the X/Y linker: a split PH domain (cyan), two SH2 domains (navy and purple), and an SH3 domain (gold). The C terminus (dotted outline) of PLC-γ is degenerate; unless otherwise noted, studies reported here used PLC-γ1 truncated at residue 1219. Lower panel, elution of PLC-γ1 from a size exclusion column and monitored by absorbance (gray line) was simultaneously analyzed with multi-angle light scattering (black line) to provide a mean molecular mass of 141.6 kDa consistent with a monomer (calculated molecular mass of 140.1 kDa). Purity of PLC-γ1 protein (2 μg, inset) was assessed by SDS-PAGE analysis followed by staining with Coomassie Brilliant Blue. B, removal of the X/Y linker constitutively activates purified PLC-γ1. Lipase activity of purified PLC-γ1 (left panel) or PLC-γ1ΔX/Y linker (right panel) was measured at the indicated protein concentrations using mixed detergent-phospholipid micelles. The indicated specific activities are the means of at least three independent experiments. Purity (2 μg, inset) of PLC-γ1 proteins was assessed by SDS-PAGE analysis followed by Coomassie Brilliant Blue staining. See also supplemental Fig. S1.