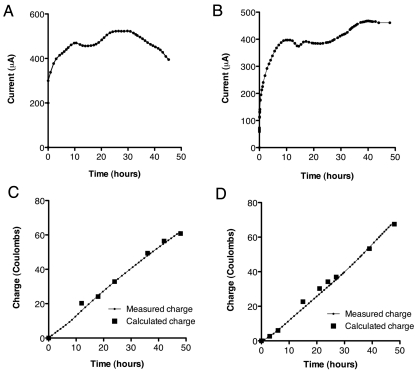
FIG 4 .
Electron balance (coulombic efficiency) for conversion of glycerol to ethanol. (A, B) Representative chronoamperometry of current produced from the conversion of glycerol to ethanol in three-electrode bioreactors (n = 3) inoculated with the wild-type strain with pGUT2PET (A) and the ∆pta strain with pGUT2PET (B). At time zero, ~1.0 OD of cells was added to the reactor. (C, D) Determining the coulombic efficiency of engineered pathways. Representative data of real-time, continuously measured charges and total calculated charges from the stoichiometric conversion of glycerol to ethanol for the wild-type strain with pGUT2PET (C) and the ∆pta strain with pGUT2PET (D). The measured charge was determined from current data in panel A or B and continuously measured during the experiment. The calculated charge is based upon the stoichiometry of the reaction mechanism and was determined when the samples used to obtain the data in panels A and B were extracted for HPLC analysis. See the text for a detailed description.