PREVIOUS reports about the use of FK 506 after renal transplantation have shown it to be associated with equivalent patient and graft survival, when compared with cyclosporine (CyA)-based regimens, and improved secondary outcomes.1-3 These latter have included lower or absent chronic steroid requirements, less need for antihypertensive medications, and lower serum cholesterol levels. On the basis of these studies, a new randomized trial was begun to compare two FK 506-based regimens, with or without azathioprine as a third agent. Although 1-year actuarial data are not yet available, enough information has been obtained to provide a preliminary summary.
MATERIALS AND METHODS
Between August 1, 1991 and May 1, 1992, 125 patients undergoing renal transplantation at the University of Pittsburgh were entered into a randomized trial comparing FK 506/prednisone and FK 506/azathioprine/prednisone. All adults undergoing kidney transplantation only, who agreed to participate in the trial, were eligible. Pediatric patients (8), patients receiving comitant multiple organs (8), and patients refusing or not recommended by their physicians to enter the trial (2) were excluded (there were, in fact, no refusals among the eligible patients).
Recipient and donor characteristics are summarized in Tables 1 and 2. Thirty-two percent of the recipients were undergoing retransplantation; 16% of the patients had a panel reactive antibody level of greater than 40%. Fourteen percent of the recipients were over 60 years of age. Seventeen percent of the cadaveric kidneys were from pediatric donors 3 years or younger and we used en bloc. These high-risk indicators have been typical of our patient profiles in recent years.
Table 1.
Recipient Characteristics
| FK 506/ Prednisone (%) |
FK 506/Azathioprine/ Prednisone (%) |
Total (%) | |
|---|---|---|---|
| No. of patients | 63 | 62 | 125 |
| Mean age (y) | 42 ± 13 | 44 ± 15 | 43 ±14 |
| Range | 19–66 | 18–78 | 18–78 |
| Age >60+ | 6 (10) | 12 (19) | 18 (14) |
| First transplant | 47 (75) | 38 (61) | 85 (68) |
| Retransplant | 16 (25) | 24 (39) | 40 (32) |
| PRA >40 | 8 (13) | 12 (19) | 20 (16) |
| Cadaver | 50 (79) | 58 (94) | 108 (86) |
| Living donor* | 13 (21) | 4 (6) | 17 (14) |
Abbreviations: PRA, panel reactive antibody.
P < .05.
Table 2.
Donor Characteristics
| FK 506/ Prednisone |
FK 506/Azathioprine/ Prednisone |
Total | |
|---|---|---|---|
| Mean age (y) | 39 ± 17 | 41 ± 18 | 40 ± 18 |
| Range | 0.3–68 | 0.3–67 | 0.3–68 |
| Mean cold ischemia time (h) |
31.9 ± 6.7 | 32.4 ± 8.0 | 32.2 ± 7.4 |
| Pediatric en bloc (%) | 9 (18) | 9 (16) | 18 (17) |
FK 506 0.15 mg/kg was given as an oral dose preoperatively. FK 506 0.1 mg/kg/d IV as a continuous infusion was begun postoperatively in the recovery room and continued until the patient was able to tolerate a diet. Oral FK 506 0.15 mg/kg twice per day was then started, with dosage adjustment based on plasma levels.
Patients received a 1000-mg bolus of IV methylprednisolone in the operating room, followed by a short steroid taper from 200 to 20 mg/d over 6 days. Further dosage reduction was individualized according to the patient’s course.
Azathioprine was started at a dose of 3 mg/kg, both preoperatively and postoperatively, with dosage adjustment based on the white blood cell count.
Rejection was documented histologically in almost all cases. Treatment generally began with a bolus of IV methylprednisolone, 1000 mg, followed by the same recycle used for induction. Steroid resistance was treated with OKT3 5 mg/d × 10 days or ATGAM. Induction OKT3 was used in one case.
RESULTS
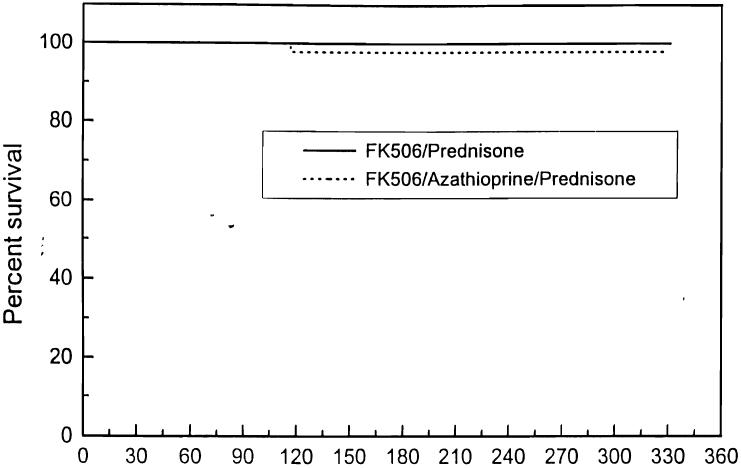
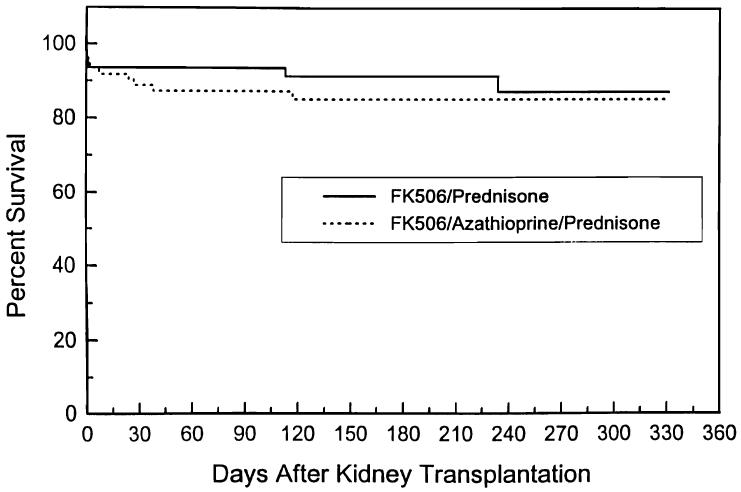
The mean follow-up is 5.5 ± 2.5 months (Tables 3-5, Figs 1, 2).
Table 3.
Results
| 6 Mo Actuarial Survival | FK 506/Prednisone | FK 506/Azathioprine/Prednisone | Total |
|---|---|---|---|
| Patient survival (%) | 100 | 98 | 99 |
| Graft survival (%) | 92 | 85 | 88 |
| Cadaveric (%) | 88 | 84 | 86 |
| Living donor (%) | 100 | 100 | 100 |
| First transplant (%) | 91 | 89 | 91 |
| Retransplant (%) | 88 | 79 | 83 |
| Mean serum creatinine | 1.9 ± 0.7mg/dL | 1.9 ± 0.7mg/dL | 1.9 ± 0.7mg/dL |
| Mean BUN | 30 ± 13 mg/dL | 31 ± 13 mg/dL | 30 ± 13 mg/dL |
| Mean cholesterol | 199 ± 46 mg/dL | 195 ± 48 mg/dL | 197 ± 47 mg/dL |
Note: Mean follow-up was 5.5 ± 2.5 months.
Table 5.
Immunosuppression
| FK 506 | FK 506/Prednisone |
FK 506/ Azathioprine/ Prednisone |
Total |
|---|---|---|---|
| Mean dose | |||
| Mg/d | 15.1 ± 8.1 | 16.4 ± 7.0 | 15.8 ± 7.6 |
| Mg/kg/d | 0.21 ± 0.11 | 0.23 ± 0.10 | 0.22 ± 0.11 |
| Level ng/mL | 1.0 ± 0.6 | 1.1 ± 0.7 | 1.1 ± 0.6 |
| Prednisone | |||
| Mean dose (mg/d) | 7.5 ± 5.6 | 8.1 ± 5.8 | 7.8 ± 5.7 |
| Off prednisone (%) | 14 (25) | 11 (21) | 25 (23) |
Note: Mean follow-up was 5.5 ± 2.5 months.
Fig 1.
Actuarial patient survival: FK 506/prednisone vs FK 506/azathioprine/prednisone.
Fig 2.
Actuarial graft survival: FK 506/prednisone vs FK 506/azathioprine/prednisone.
Mortality
Of the first 125 cases, one patient has died; this was a 50-year-old white woman 4 months after a second transplant who died of colonic perforation and Pseudomonas sepsis. The 6-month actuarial patient survival is 99%.
Graft Survival
The 6-month actuarial graft survival is 88%; 110 (88%) of the 125 patients have functioning kidneys, Ten (8%) of patients are considered to have lost their grafts, 4 to rejection, 3 to technical reasons, 2 to disease recurrence, and 1 to patient death. There are 5 (4%) patients who have had significant rejection but may yet have recovery of graft function. Ninety-one percent of patients undergoing their first or second transplant have functioning grafts, as do 86% of cadaveric and 100% of living donor recipients. The mean serum creatinine and blood urea nitrogen (BUN) are 1.9 ± 0.7 mg/dL and 30 ± 13 mg/dL. No difference was seen between the two-drug and three-drug groups in patient or graft survival and quality of graft function.
Rejection
The overall incidence of rejection was 46%; 13% of patients required OKT3 or ATGAM for steroid-resistant rejection. Fifty-one percent of two-drug and 40% of the three-drug group experienced rejection (P = NS); 16% of the two-drug group and 10% of the three-drug group required OKT3 (P = NS).
CMV
Cytomegalovirus (CMV) infection or disease was present in 13% of recipients and was treated with IV ganciclovir. All patients routinely received high-dose acyclovir prophylaxis; CMV seronegative recipients of seropositive kidneys also received CMV immunoglobulin (Cytogam).
Initial Nonfunction
Thirty-seven percent of the recipients required dialysis at least once during the first week after transplantation.
New Onset of Diabetes Mellitus
Eleven percent of the nondiabetic recipients had at least temporary need for insulin after transplantation. This percentage appeared to be greater in the two-drug (16%) group than in the three-drug (6%) group, but was not statistically different.
Crossover
Twenty-six percent of the three-drug group had discontinuation of azathioprine at some point after transplantation, while 24% of the two-drug group required the addition of azathioprine to control persistent rejection.
Posttransplant Lymphoproliferative Disorder (PTLD)
PTLD was seen in the renal biopsy of one (1%) patient on two-drug immunosuppression. The patient was otherwise asymptomatic and had a normal computed tomographic (CT) scan. This was treated with high-dose IV acyclovir and cessation of FK 506 and prednisone.
Cholesterol
Mean serum cholesterol was 197 ± 47 mg/dL; it was 199 ± 46 mg/dL in the two-drug group and 195 ± 48 mg/dL in the three-drug group.
Antihypertensive Medications
Thirty-two (29%) patients are taking no antihypertensive medications while 40 (36%) are on one, 27 (25%) are on two, 10 (9.1%) are on three, and 1 (1%) is on four antihypertensive medications. There was no difference between the two-drug and three-drug groups (Tables 6 and 7).
Table 6.
Steroid Requirements
| Prednisone Dose | FK 506/ Prednisone (%) |
FK 506/Azathioprine/ Prednisone (%) |
Total (%) |
|---|---|---|---|
| 0 | 14 (25) | 11 (21) | 25 (23) |
| 2.5–5.0 mg/d | 9 (16) | 8 (15) | 17 (16) |
| 7.5–100 mg/d | 21 (37) | 20 (38) | 41 (37) |
| 12.5–15.0 mg/d | 9 (16) | 9 (17) | 18 (17) |
| 17.5–20.0 mg/d | 4 (7) | 5 (9) | 9 (8) |
Table 7.
Antihypertensive Requirements
| Number | FK 506/ Prednisone (%) |
FK 506/Azathioprine/ Prednisone (%) |
Total (%) |
|---|---|---|---|
| 0 | 18 (31) | 14 (26) | 32 (29) |
| 1 | 17 (30) | 23 (43) | 40 (36) |
| 2 | 15 (26) | 12 (23) | 27 (25) |
| 3 | 6 (11) | 4 (8) | 10 (9) |
| 4 | 1 (2) | 0 (0) | 1 (1) |
Steroid Requirements
Twenty-five (23%) patients have been taken off prednisone. Seventeen (10%) are on 2.5 to 5 mg/d, while 41 (37%) are on 7.5 to 10 mg/d, and 27 (25%) are taking 12.5 to 20 mg/d (Tables 6 and 7). The mean daily prednisone doses are 7.8 ± 5.7 mg. The two- and three-drug groups behave similarly.
FK 506
The mean FK 506 dosage is 15.8 ± 7.6 mg/d (0.22 ± 0.11 mg/kg/d) and is similar between the two groups. The mean FK 506 level is 1.1 ± 0.6 ng/mL.
DISCUSSION
Previous studies of FK 506 in kidney transplantation have shown it to be equally efficacious when compared with CyA-based regimens.1-3 This randomized trial was started to look at the effect of a third agent, azathioprine, on patient and graft survival, quality of renal function, and incidence of rejection, CMV, and other complications. Thus far, there is little evidence for a major difference between the two-drug and three-drug regimens, although there may be a trend toward less rejection and less diabetes in the three-drug group. The need for crossover from the two-drug to the three-drug group in nearly one fourth of the cases suggests that there is a subset of patients who require a third agent. There is, however, an equally high incidence of crossover from the three-drug to the two-drug group. Thus, there may be some utility for a third drug with FK 506 in renal transplantation; it is unclear whether azathioprine is the ideal candidate.
Overall, the results suggest an improved outcome in patient and graft survival, compared with the earlier experience with the drug. While the reasons are not clear, it may be that there is a significant learning curve with FK 506. There is certainly a need to achieve a balance in dosage to avoid either rejection or nephrotoxicity. Graft biopsies are frequently necessary. Thus far, a relatively low incidence of need for OKT3, CMV, PTLD, and diabetes mellitus has been observed, although the incidence of rejection is still significant.
It is hoped that this encouraging report will stimulate interest in further trials of FK 506 in kidney transplantation.
SUMMARY
FK 506 was used as a primary immunosuppressive agent in 125 cases of renal transplantation in a randomized trial comparing FK 506/prednisone with FK 506/azathioprine/prednisone. With a mean follow-up of 5.5 ± 2.5 months, there has been a 6-month actuarial patient survival of 99% and graft survival of 88%. There is no difference thus far between the two-drug and three-drug groups, although there may be less rejection and diabetes in the three-drug group. These results suggest that FK 506 is a useful immunosuppressive agent in kidney transplantation.
Table 4.
Complications
| FK 506/ Prednisone (%) |
FK 506/Azathioprine/ Prednisone (%) |
Total (%) |
|
|---|---|---|---|
| Rejection | 32 (51) | 25 (40) | 57 (46) |
| OKT3/ATGAM | 10 (16) | 6 (10) | 16 (13) |
| CMV | 8 (13) | 8 (13) | 16 (13) |
| PTLD | 1 (2) | 0 (0) | 1 (1) |
| New onset diabetes | 7 (16) | 3 (6) | 10 (11) |
Note: Mean follow-up was 5.5 ± 2.5 months.
ACKNOWLEDGMENTS
We would like to thank Regina Fenton, RN, BSN, CCTC, Loraine Kaminski, RN, Joan Murray, RN, BSN, CCTC, Deborah Good, RN, CCTC, Holly Woods, RN, Marie Hawranko, RN, BSN, Jareen Flohr, RN, BSN, Sue Bauder, RN, and Janice Zagari, RN, BSN in their help with patient care and management; Dan Dziak, BSc, Janet Schmeltzer, and Yvette Carey for their help with data collection and organization; and Karen Blair for her help with manuscript preparation.
REFERENCES
- 1.Starzl TE, Fung JJ, Jordan M, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro R, Jordan M, Fung JJ, et al. Transplant Proc. 1991;23:920. [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro R, Jordan ML, Scantlebury V, et al. Transplant Proc. 1991;23:3065. [PMC free article] [PubMed] [Google Scholar]