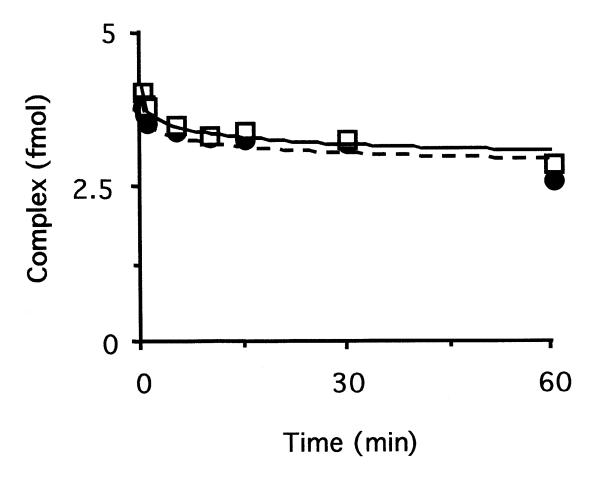
Figure 7.
Dissociation rates of the complex between hOGG1 and DNA containing an incision 3′ of an AP site. The complex between GST–hOGG1 and its AP lyase activity product was obtained by incubation of an 8-oxoG-containing oligonucleotide (10 fmol) with 215 fmol of GST–hOGG1 fusion protein. After confirming the complete cleavage of the substrate, a 100-fold excess of unlabelled (8-oxoG:C) oligonucleotide was added, in the absence (circles) or presence (squares) of 2.5 pmol of HAP1 (time = 0). The fraction of labelled DNA present in the complex was determined by EMSA.