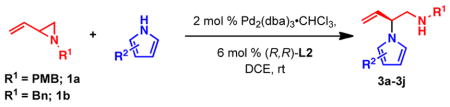
Table 2.
Reaction scope of pyrroles with vinyl aziridinesa
 | ||
|---|---|---|
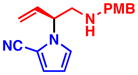 3a 99% yield, 89% ee |
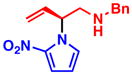 3b 77% yield, 89% ee |
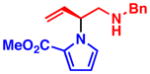 3c 76% yield, 96% ee |
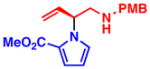 3d 86% yield, 94% ee |
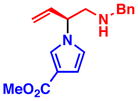 3e 86% yield, 94% ee |
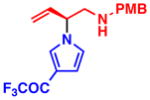 3f 92% yield, 93% ee |
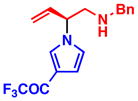 3g 95% yield, 93% ee |
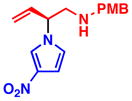 3h 85% yield, 90% eeb |
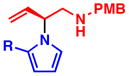 3i, R = Et: NR 3j, R = H: NR |
All reactions were performed with 1.0 equiv of pyrrole nucleophile and 1.1 equiv of 1 at ambient temperature. Isolated yield. % ee was determined by chiral HPLC.
1.1 equiv of Cs2CO3 was added.
