Table 4.
Reactions of nitrogen heterocycles with vinyl aziridines to access piperazinonesa
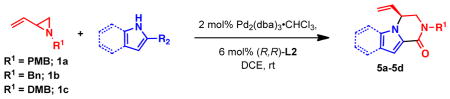 | |||
|---|---|---|---|
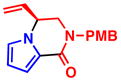 5a, R2 = COCF3 97% yield, 90% eeb |
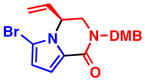 5b, R2 = CO2Me 72% yield, 95% eec |
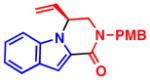 5c, R2 = CO2Me 72% yield, 93% ee |
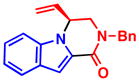 5d, R2 = CO2Me 97% yield, 96% ee |
All reactions were performed with 1.0 equiv of heterocyclic nucleophile and 1.1 equiv of 1 at ambient temperature. Isolated yield. % ee was determined by chiral HPLC.
1.1 equiv of Cs2CO3 was added.
Performed using 2.5 mol % [Pd(C3H5)Cl]2 7.5 mol % (R,R)-L1, in CH2Cl2 at rt.
