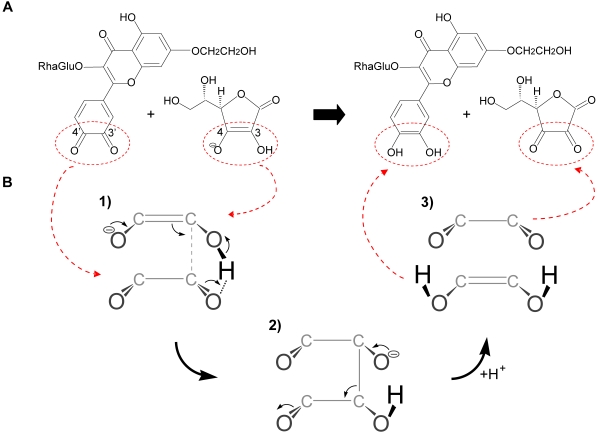
Figure 6. Chemical reaction of oxidized monoHER with ascorbate.
(A) Chemical structure of oxidized monoHER (left) and ascorbate (right). The active part of ascorbate and the active part of oxidized monoHER are indicated by the red ellipses. (B) Suggested route for the reaction of oxidized monoHER with ascorbate. Only the active parts are shown to illustrate the suggested mechanism more clearly. (1) The active part of ascorbate (top) approaches the active part of oxidized monoHER (bottom) due to a π-π interaction and a hydrogen bond. The π-electrons of ascorbate are used to create a new bond. The C3 of ascorbate will most likely attack the C3′ of the monoHER quinone because it is more electron deficient than the C4′ according to Spartan ‘06. (2) After the attack, a transition state, with an sp3 bond between ascorbate and oxidized monoHER, is suggested to be formed. (3) This intermediate rapidly decomposes into monoHER and oxidized ascorbate. The driving force of this reaction is the restoration of the highly conjugated π-system of monoHER.