Abstract
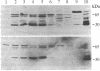
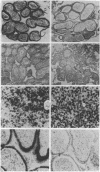
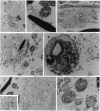
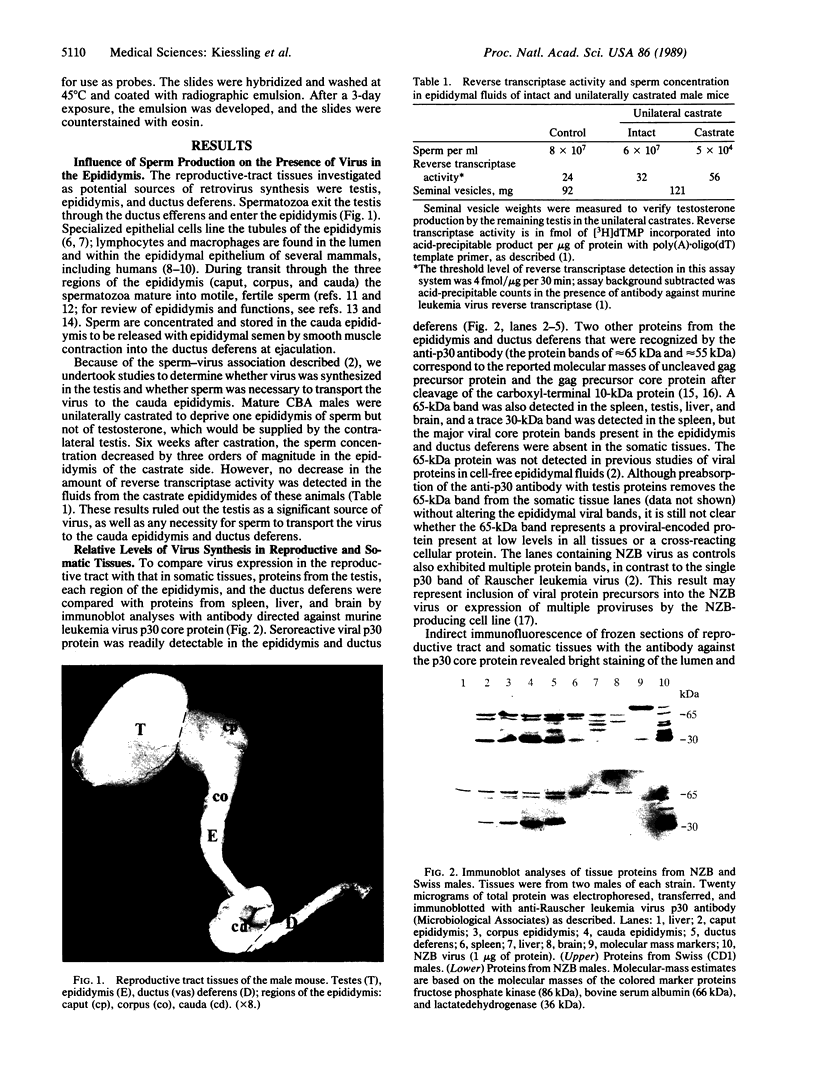
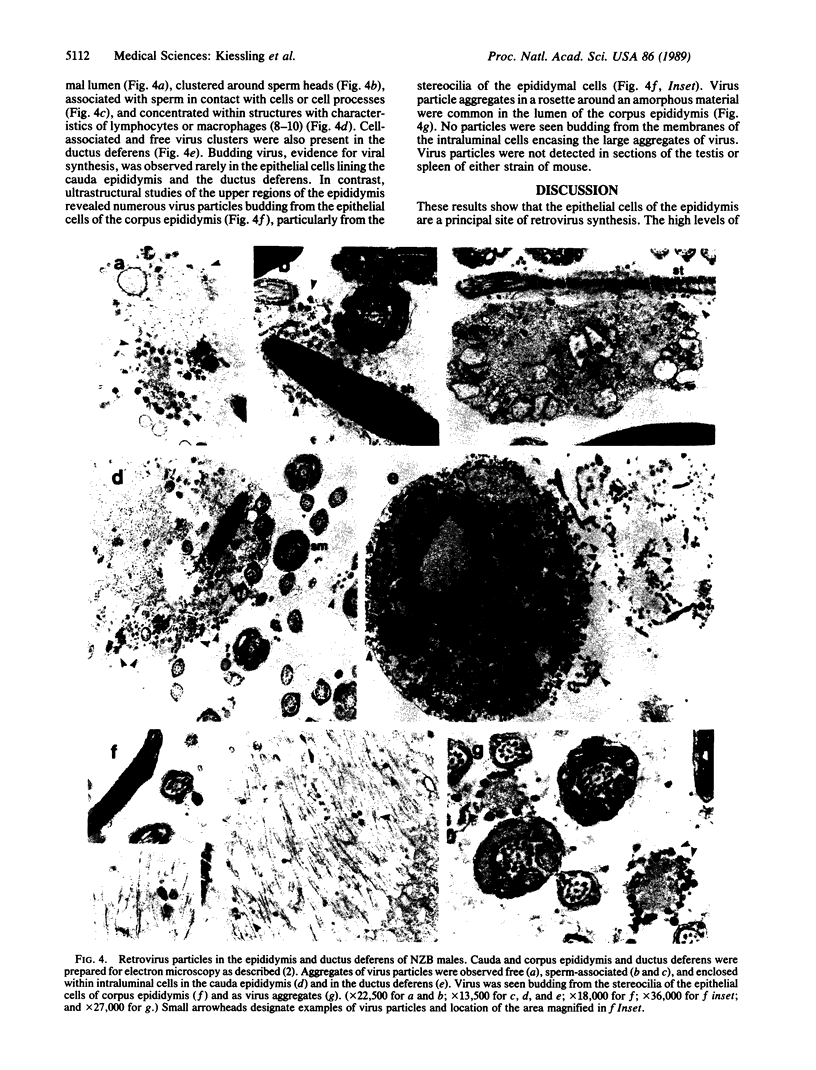
High levels of retrovirus particles are present in the reproductive tract of male mice. In this report epithelial cells that line the lumen of the epididymis are shown to be a principal site of virus synthesis. Aggregates of free virus were evident in the epididymal lumen in addition to the sperm-associated virus previously reported. Large intraluminal cells with characteristics of macrophages and engorged with virus particles were also seen. Virus particles were not detected in testis, liver, brain, or spleen. Thus, the epididymal epithelium is a principal reservoir for retrovirus expression. The virus would be ejaculated as free, cell-associated, and sperm-bound particles. The high level of expression and the relative isolation of epididymal virus from the immune system may relate to venereal transmission of retrovirus infections in mice and humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Takano H., Ito T. Ultrastructure of the mouse epididymal duct with special reference to the regional differences of the principal cells. Arch Histol Jpn. 1983 Feb;46(1):51–68. doi: 10.1679/aohc.46.51. [DOI] [PubMed] [Google Scholar]
- Abou-Haïla A., Fain-Maurel M. A. Regional differences of the proximal part of mouse epididymis: morphological and histochemical characterization. Anat Rec. 1984 Jun;209(2):197–208. doi: 10.1002/ar.1092090207. [DOI] [PubMed] [Google Scholar]
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Ashida E. R., Scofield V. L. Lymphocyte major histocompatibility complex-encoded class II structures may act as sperm receptors. Proc Natl Acad Sci U S A. 1987 May;84(10):3395–3399. doi: 10.1073/pnas.84.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzy M. S., Connell R. S., Kiessling A. A. Detection of human immunodeficiency virus in cell-free seminal fluid. J Acquir Immune Defic Syndr. 1988;1(5):419–424. [PubMed] [Google Scholar]
- Brooks D. E. Secretion of proteins and glycoproteins by the rat epididymis: regional differences, androgen-dependence, and effects of protease inhibitors, procaine, and tunicamycin. Biol Reprod. 1981 Dec;25(5):1099–1117. doi: 10.1095/biolreprod25.5.1099. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Chiri A., Dougherty M. F., Casagrande J., Estes J. D. Congenital transmission of murine leukemia virus from wild mice prone to the development of lymphoma and paralysis. J Natl Cancer Inst. 1979 Jan;62(1):63–70. [PubMed] [Google Scholar]
- Hoffer A. P., Hamilton D. W., Fawcett D. W. The ultrastructure of the principal cells and intraepithelial leucocytes in the initial segment of the rat epididymis. Anat Rec. 1973 Feb;175(2):169–201. doi: 10.1002/ar.1091750205. [DOI] [PubMed] [Google Scholar]
- Kiessling A. A., Crowell R. C., Connell R. S. Sperm-associated retroviruses in the mouse epididymis. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8667–8671. doi: 10.1073/pnas.84.23.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling A. A. Evidence that reverse transcriptase is a component of murine epididymal fluid. Proc Soc Exp Biol Med. 1984 Jun;176(2):175–182. doi: 10.3181/00379727-176-41859. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Joyner J., Borenfreund E. Mouse sperm can horizontally transmit type C viruses. J Gen Virol. 1980 Dec;51(Pt 2):439–443. doi: 10.1099/0022-1317-51-2-439. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Kazan P., Varnier O., Kleiman H. Murine xenotropic type C viruses I. Distribution and further characterization of the virus in NZB mice. J Virol. 1975 Oct;16(4):844–853. doi: 10.1128/jvi.16.4.844-853.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgebin-Crist M. C. Sperm maturation in rabbit epididymis. Nature. 1967 Nov 25;216(5117):816–818. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- Overstreet J. W., Bedford J. M. Transport, capacitation and fertilizing ability of epididymal spermatozoa. J Exp Zool. 1974 Aug;189(2):203–214. doi: 10.1002/jez.1401890208. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Hayes S. F. Horizontal transmission of murine retroviruses. J Virol. 1987 Apr;61(4):1037–1044. doi: 10.1128/jvi.61.4.1037-1044.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Clavier R., Harrison R. G., Macmillian E. W. The pattern of the lymphatic drainage of the rat epididymis. J Anat. 1982 Jun;134(Pt 4):667–675. [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985 Jan 1;161(1):72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Suzuki F. Microvasculature of the mouse testis and excurrent duct system. Am J Anat. 1982 Apr;163(4):309–325. doi: 10.1002/aja.1001630404. [DOI] [PubMed] [Google Scholar]
- Tezón J. G., Vazquez M. H., Piñeiro L., de Larminat M. A., Blaquier J. A. Identification of androgen-induced proteins in human epididymis. Biol Reprod. 1985 Apr;32(3):584–590. doi: 10.1095/biolreprod32.3.584. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Holstein A. F. Intraepithelial lymphocytes and macrophages in the human epididymis. Cell Tissue Res. 1983;233(3):517–521. doi: 10.1007/BF00212221. [DOI] [PubMed] [Google Scholar]