Fig. 4.
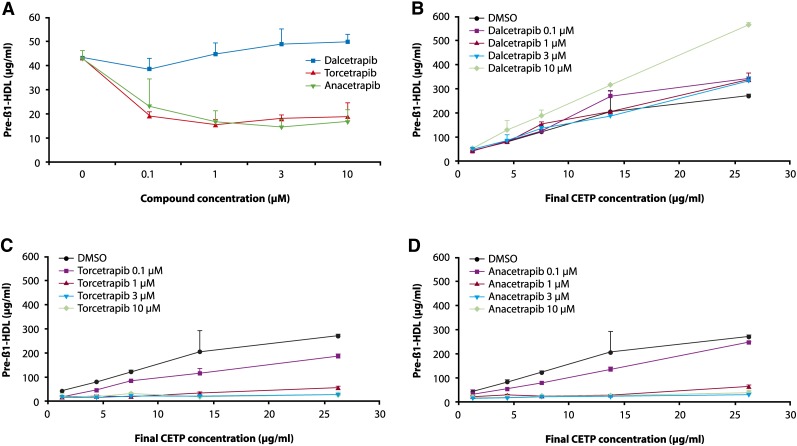
A: Human plasma with an endogenous CETP level of 1.25 μg/ml was incubated for 21 h with and without test compounds, dalcetrapib, torcetrapib, and anacetrapib (0.1, 1, 3, and 10 µM). Dalcetrapib 1, 3, and 10 µM dose-dependently increased pre-β-HDL, as quantified by ELISA, by 4, 13, and 16% (NS, P < 0.05, and 0.01, respectively). Human plasma with added recombinant human CETP (final concentration 4.38, 7.5, 13.75, and 26.25 μg/ml) was incubated for 21 h with and without test compounds (0.1, 1, 3, and 10 µM). B: Dalcetrapib significantly increased pre-β-HDL formation at concentrations of 0.1, 1, 3, and 10 µM (P < 0.001, 0.001, 0.05, and 0.001, respectively). C: Torcetrapib significantly and dose-dependently decreased pre-β-HDL formation at all concentrations tested (P < 0.01 at 0.1 µM and P < 0.001 at 1, 3, and 10 µM). D: Anacetrapib significantly decreased pre-β-HDL formation at concentrations of 1, 3, and 10 µM (each P < 0.001). Error bars represent the SD of four replicates.