Abstract
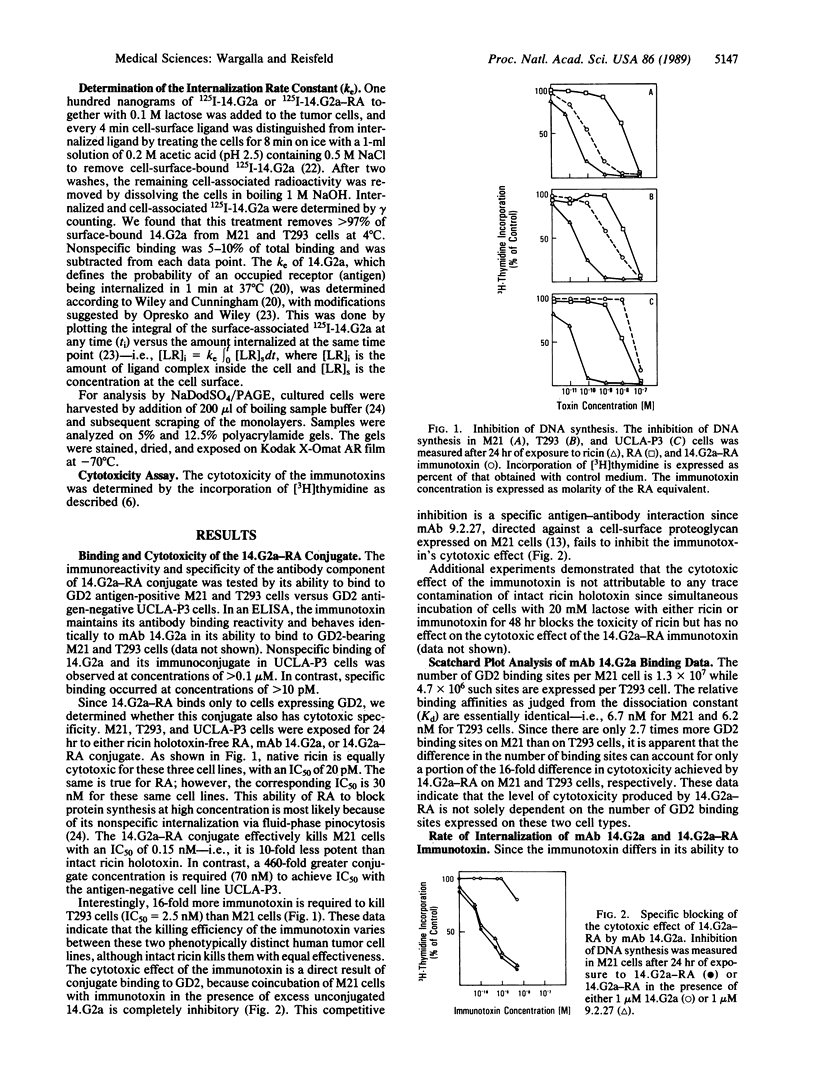
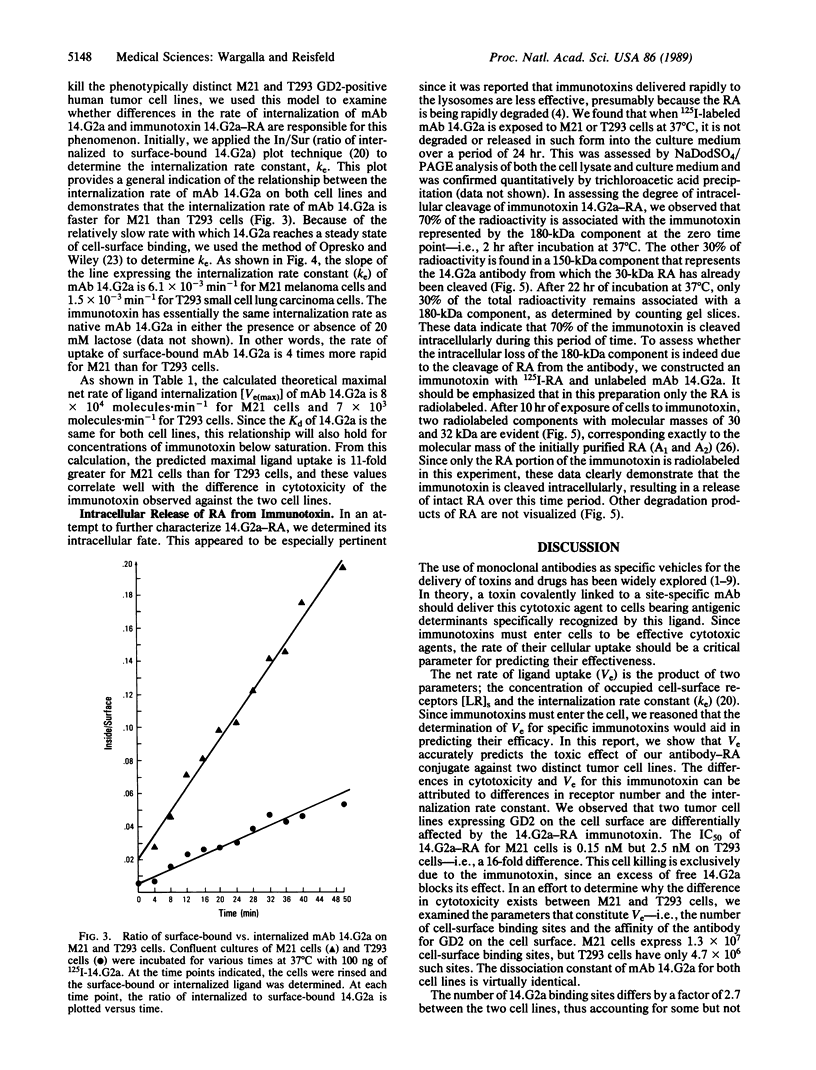
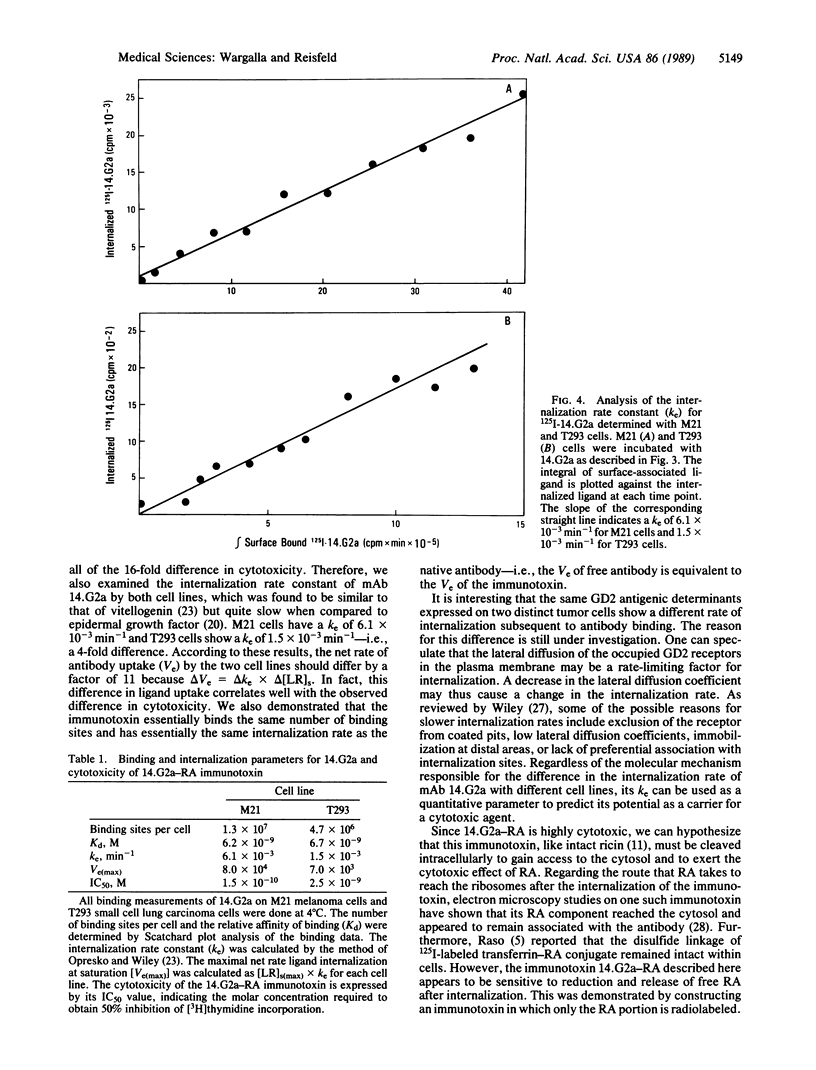
The relationship between the cellular internalization of an anti-ganglioside GD2 monoclonal antibody (14.G2a) and the toxic effect of its ricin A-chain immunotoxin (14.G2a-RA) was examined on GD2-bearing M21 human melanoma and T293 small cell lung carcinoma cell lines. The capacity for ligand uptake was determined by examining the parameters that contribute to this constant, including the number of cell-surface binding sites and the internalization rate constant (ke). The maximum uptake of 14.G2a is 11-fold greater for M21 than for T293 cells, due to a 2.7-fold difference in binding sites and a 4-fold difference in the rate of antibody internalization. The capacity for ligand uptake correlates with the cytotoxic activity of the 14.G2a-RA immunotoxin against these two cell lines. Furthermore, we were able to demonstrate that the consequence of internalization of 14.G2a-RA is the intracellular release of undegraded ricin A-chain from the antibody. These studies indicate that the rate of internalization is a quantitative parameter that plays a key role in predicting the cytotoxic potency of this immunotoxin.
Full text
PDF

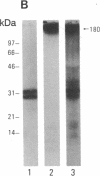
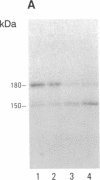
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Molthoff C., Janssen H., Hilkens J. Endocytosis and intracellular routing of an antibody-ricin A chain conjugate. Cancer Res. 1988 Jul 1;48(13):3822–3827. [PubMed] [Google Scholar]
- Casellas P., Bourrie B. J., Gros P., Jansen F. K. Kinetics of cytotoxicity induced by immunotoxins. Enhancement by lysosomotropic amines and carboxylic ionophores. J Biol Chem. 1984 Aug 10;259(15):9359–9364. [PubMed] [Google Scholar]
- Domingo D. L., Trowbridge I. S. Transferrin receptor as a target for antibody-drug conjugates. Methods Enzymol. 1985;112:238–247. doi: 10.1016/s0076-6879(85)12020-3. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem. 1988 Jun 25;263(18):8735–8739. [PubMed] [Google Scholar]
- Fulton R. J., Blakey D. C., Knowles P. P., Uhr J. W., Thorpe P. E., Vitetta E. S. Purification of ricin A1, A2, and B chains and characterization of their toxicity. J Biol Chem. 1986 Apr 25;261(12):5314–5319. [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Knowles P. P., Thorpe P. E. Purification of immunotoxins containing ricin A-chain and abrin A-chain using blue sepharose CL-6B. Anal Biochem. 1987 Feb 1;160(2):440–443. doi: 10.1016/0003-2697(87)90073-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Senter P. D., Yau-Young A., Blättler W. A., Goldmacher V. S. Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem. 1985 Oct 5;260(22):12035–12041. [PubMed] [Google Scholar]
- Mujoo K., Cheresh D. A., Yang H. M., Reisfeld R. A. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987 Feb 15;47(4):1098–1104. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973 Jul 31;12(16):3121–3126. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- Opresko L. K., Wiley H. S. Receptor-mediated endocytosis in Xenopus oocytes. II. Evidence for two novel mechanisms of hormonal regulation. J Biol Chem. 1987 Mar 25;262(9):4116–4123. [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Pirker R., FitzGerald D. J., Hamilton T. C., Ozols R. F., Laird W., Frankel A. E., Willingham M. C., Pastan I. Characterization of immunotoxins active against ovarian cancer cell lines. J Clin Invest. 1985 Sep;76(3):1261–1267. doi: 10.1172/JCI112082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press O. W., Martin P. J., Thorpe P. E., Vitetta E. S. Ricin A-chain containing immunotoxins directed against different epitopes on the CD2 molecule differ in their ability to kill normal and malignant T cells. J Immunol. 1988 Dec 15;141(12):4410–4417. [PubMed] [Google Scholar]
- Raso V., Basala M. A highly cytotoxic human transferrin-ricin A chain conjugate used to select receptor-modified cells. J Biol Chem. 1984 Jan 25;259(2):1143–1149. [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Krolick K. A., Miyama-Inaba M., Cushley W., Uhr J. W. Immunotoxins: a new approach to cancer therapy. Science. 1983 Feb 11;219(4585):644–650. doi: 10.1126/science.6218613. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Youle R. J., Neville D. M., Jr Kinetics of protein synthesis inactivation by ricin-anti-Thy 1.1 monoclonal antibody hybrids. Role of the ricin B subunit demonstrated by reconstitution. J Biol Chem. 1982 Feb 25;257(4):1598–1601. [PubMed] [Google Scholar]
- van Deurs B., Tønnessen T. I., Petersen O. W., Sandvig K., Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986 Jan;102(1):37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]