Figure 10.
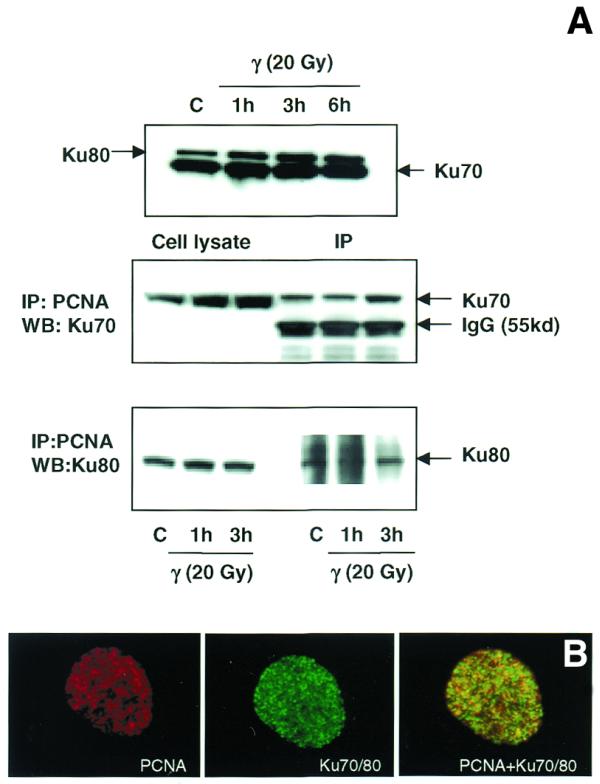
(A) Immunoblot analysis of the association of PCNA complex with Ku70/80 heterodimer by immunoprecipitation. Total cellular proteins were isolated from control and γ-irradiated (20 Gy) WI38 cells. Western blot analysis of Ku70 and Ku80 proteins in control and irradiated cells is shown in the top panel. Protein (500 µg) was immunoprecipitated using agarose-conjugated PCNA antibody (see Materials and Methods). The immunoprecipitated complex was size fractionated by 4–20% SDS–PAGE and transferred to PVDF membrane. The membranes were immunoreacted with mouse monoclonal antibodies to Ku70 (middle panel) and Ku80 (lower panel) and the signal was detected using the ECL kit. Ku70 antibody detected a cross-reacting immunoglobulin band of 55 kDa (middle panel). (B) Immunological co-localization of PCNA and Ku70/80 heterodimer in the interphase nucleus after γ-irradiation. WI38 cells in G1 phase were irradiated with 10 Gy of γ-rays, post-incubated for 3 h and fixed in acetone:methanol (1:1) after extraction with hypotonic buffer. The cells were sequentially immunostained for PCNA (detected by Texas red-conjugated secondary antibody to mouse IgG) and Ku70/80 heterodimer (detected by FITC-conjugated secondary antibody to mouse IgG). The immunostaining of PCNA (red), Ku70/80 heterodimer (green) and the merging of both PCNA and Ku70/80 heterodimer (yellow) are shown.
