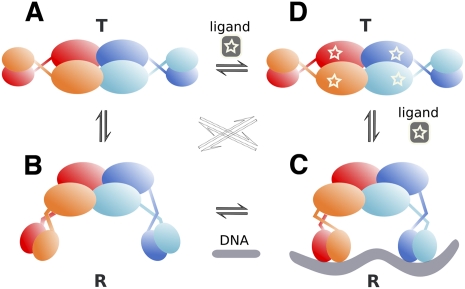
Figure 6.
A proposed cooperative binding and induction mechanism for TtgV. TtgV exists in equilibrium of a straight linker helix (symmetric T-state) (A) or bent/flexible linker helix (asymmetric R-state) (B). The T-state is more stable, while the R-state favors DNA binding. (C) Upon DNA binding, the favorable interaction energy between the protein and the DNA stabilizes the unstable R-state. (D) Upon effector binding, the CTDs slide back to the symmetric configuration and the DBDs rotate in opposite directions, releasing from DNA and returning to the stable symmetric configuration (T-state).