Abstract
The triazole antifungal pramiconazole (Stiefel, a GSK company) was compared with itraconazole, miconazole, and terbinafine in vitro and in vivo. Potent in vitro activities against Candida spp. (50% inhibitory concentration [IC50], 0.04 to 1.83 μM) and Microsporum and Trichophyton spp. (IC50, 0.15 to 1.34 μM) were obtained but not, however, against other filamentous molds and zygomycetes. In the M. canis guinea pig model and C. albicans vulvovaginitis rat model, pramiconazole was superior to the reference compounds after oral and topical administration.
Although considerable research is invested in finding novel strategies for the treatment of fatal invasive mycoses (6), nonfatal superficial mycoses believed to infect about 25% of the world population should not be overlooked (1). The most widespread dermatomycoses are caused by Trichophyton, Epidermophyton, and Microsporum species. Treatment is oral or topical with the allylamine terbinafine or any of the azoles (4, 13, 14). Yeasts also cause superficial infections of skin and mucous membranes, whereby vulvovaginal candidiasis (VVC) affects at least 75% of all women at least once in their lives (15, 21). Standard therapy involves intravaginal application of clotrimazole or miconazole or oral treatment with fluconazole or itraconazole (15). Although current treatment options may suffice, new antifungals would still be acceptable to improve treatment compliance or reduce adverse effects and drug interactions. The triazole pramiconazole shows good in vitro and clinical activity against dermatophytes and yeasts (12, 16, 17) and Malassezia infections (10, 11, 19). Laboratory data always refer to oral treatment of mice and guinea pigs (16, 17); however, no data on topical application are available. No data have yet been published on pramiconazole in VVC in comparison with reference drugs, although it is in clinical de-velopment for these indications (8, 9). The specific aims of this laboratory study were (i) to perform an in vitro profiling of pramiconazole and (ii) to evaluate oral and topical treatment schemes against Microsporum canis in guinea pigs and Candida albicans VVC in rats.
Miconazole (MC), itraconazole (ITC), and terbinafine (TRB) were purchased from Sigma, while pramiconazole (PRC) was provided by Stiefel-GSK. The fungal isolates were obtained from the Scientific Institute of Public Health (IHEM, Brussels, Belgium) and cultivated on Sabouraud dextrose agar (SDA) (Oxoid). For all species, a stock of 5 × 106 CFU/ml was prepared in RPMI-MOPS medium with 10% glycerol and stored in liquid nitrogen for later use in all in vitro tests. Fresh inocula were used for animal infections. The in vitro susceptibility screens were performed as previously described (7). Briefly, 10 μl of prediluted compound solution was spotted onto 96-well plates (U-bottom; Greiner Bio-One) with 64 μM as the highest concentration; 103 CFU in 200 μl RPMI-MOPS was added to each well. After incubation, growth inhibition was measured after adding 10 μl/well 0.005% (wt/vol) resazurin (Sigma), allowing fluorimetric reading (λex, 550 nm; λem, 590 nm) (23). Activity is expressed as IC50, i.e., the concentration that inhibits growth for 50% compared to nontreated controls. Cytotoxicity was simultaneously tested on human lung fibroblasts (MRC-5SV40) (Invitrogen). Five independent replicates were performed for each observation.
The in vitro IC50s for reference drugs were comparable to the ranges in literature (2, 5, 20), and available data on pramiconazole were also confirmed (16, 17) (Table 1). TRB performs marginally better against dermatophytes. Except for Trichophyton quinckeanum, PRC activity remained below 0.5 μM. Against Candida spp., activities remained below 1 μM, except for C. albicans B2630. PRC failed to show activity (IC50, >64 μM) against the other filamentous molds and zygomycetes (data not shown).
TABLE 1.
Cytotoxicity (CC50) and activity (IC50) against four dermatophyte and four Candida species
| Human cell or fungal isolate | Activitya ± SD |
|||
|---|---|---|---|---|
| TRB | MC | ITC | PRC | |
| Cells | ||||
| MRC-5 | 63.00 ± 1.73 | 29.67 ± 13.32 | 49.33 ± 14.50 | 53.33 ± 18.48 |
| Fungal isolates | ||||
| Microsporum canis B68128 | 0.10 ± 0.05 | 0.23 ± 0.16 | 2.02 ± 2.45 | 0.18 ± 0.06 |
| Trichophyton mentagrophytes B70554 | 0.06 ± 0.04 | 0.40 ± 0.28 | 0.37 ± 0.39 | 0.15 ± 0.16 |
| T. rubrum B68183 | 0.07 ± 0.05 | 0.33 ± 0.26 | 0.56 ± 0.48 | 0.35 ± 0.14 |
| T. rubrum J941704 | 0.03 ± 0.02 | 0.14 ± 0.09 | 0.98 ± 1.31 | 0.19 ± 0.19 |
| T. quinckeanum B68683 | 0.01 ± 0.01 | 0.79 ± 0.49 | 2.93 ± 2.91 | 1.34 ± 1.21 |
| Candida albicans B59163 | 3.57 ± 1.39 | 0.30 ± 0.22 | 1.41 ± 1.20 | 0.04 ± 0.03 |
| C. albicans B2630 | 64.00 ± 0.00 | 2.50 ± 2.08 | 1.39 ± 1.86 | 1.83 ± 2.34 |
| C. glabrata B63155 | 30.66 ± 23.68 | 0.12 ± 0.06 | 3.74 ± 4.63 | 0.65 ± 0.15 |
| C. kefyr B46120 | 6.33 ± 4.80 | 0.03 ± 0.03 | 0.40 ± 0.35 | 0.13 ± 0.06 |
| C. krusei B68404 | 64.00 ± 0.00 | 1.40 ± 0.57 | 4.21 ± 4.90 | 0.87 ± 0.50 |
For MRC-5 cells, the CC50 (the concentration at which 50% of the MRC-5 cells are killed) is given. For fungal isolates, the IC50 (the concentration in μM at which growth is inhibited for 50% compared to untreated controls) is given. Averages of five independent repeats are expressed, together with the standard deviation (SD). Conversion factors to be used for the IC50s in μg/ml: TRB, × 0.33; MC, × 0.42; ITC, × 0.70; PRC, × 0.66.
All animal experiments were approved by the Ethical Commission of the University of Antwerp (2008/015). Compounds were formulated in polyethylene glycol 200 (PEG200) for oral (PO) dosing and in PEG400-1,500 (3:2, wt/wt) for topical (TP) administration. Each treatment was evaluated for six animals grouped into two independent experiments. Group averages of lesion scores (LS) or intravaginal burdens were used to plot graphs, and the area under the infection curve (AUC) was calculated for each animal as a measure for infection burden. An unpaired t test (two tailed, P ≤ 0.05) was used to determine levels of significance between the different experimental groups.
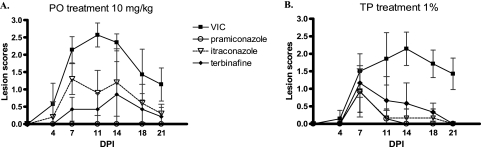
The dorsum of each female guinea pig was shaved and scarified with a steel brush. An inoculum (M. canis B68128) of 106 CFU in 100 μl was applied to the scarified skin. Oral dosing at 10 mg/kg started about 2 h before infection and was continued for 5 days. Topical treatment using a 1% formulation was applied twice daily for 4 days starting on the morning after infection. Skin lesions were evaluated every 3 to 4 days (Fig. 1). Lesion scoring systems as found in the literature (18, 24) were slightly modified to include both lesion size and severity. Upon oral administration at 10 mg/kg (Fig. 1A), PRC performed much better than ITC and TRB, with complete suppression of lesion development, which contrasts with the in vitro data in which TRB was better than PRC (P = 0.004) and ITC (P = 0.005). The latter can be explained by the better pharmacokinetic properties of PRC (Table 2), the lower protein binding (17) and higher metabolic stability (3). After TP application, PRC was better than TRB (P = 0.041), but no difference was observed between PRC and ITC (Fig. 1B).
FIG. 1.
Comparative efficacy of pramiconazole, terbinafine, and itraconazole after oral (PO) dosing (once daily [s.i.d.]) at 10 mg/kg (A) and after topical (TP) treatment (twice daily [b.i.d.]) with 1% (wt/wt) cream (B) against M. canis in guinea pigs. The scores assigned to the animals are shown on the y axis; the x axis represents the days after infection. VIC, vehicle-treated control group; DPI, days postinfection.
TABLE 2.
Pharmacokinetics (PK) after oral administration of pramiconazole, itraconazole, and terbinafine to guinea pigs
| PK parameter | Result with: |
||
|---|---|---|---|
| PRCb | ITCc | TRBd | |
| Cmax (μg/ml)a | 0.18 | 0.35 | 0.06 |
| Tmax (min) | 240 | 120 | 42 |
| t1/2 (h) | 23 | 13.9 | 6.6 |
Plasma concentrations were normalized to a dose of 10 mg/kg.
40 mg/kg; source, Janssen Pharmaceutica, unpublished data.
20 mg/kg; source, Sobue et al. (22).
10 mg/kg; source, Janssen Pharmaceutica, unpublished data.
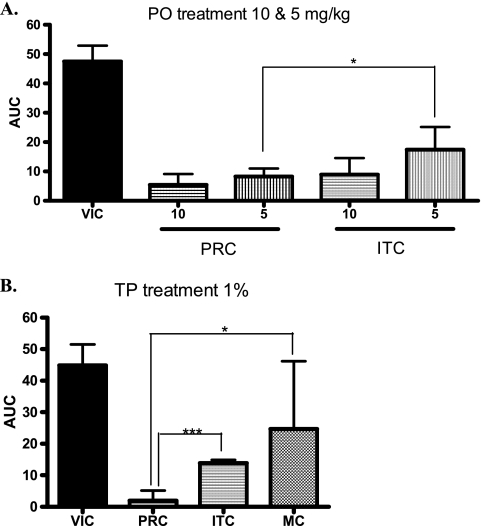
For VVC, female rats were ovariectomized 3 weeks before infection and estrus was induced with 1 mg estradiol-benzoate plus 200 μg progesterone on days −3, 2, and 7. Rats were infected intravaginally with 107 CFU C. albicans B2630. Treatment schedules were identical to those of the guinea pig model. At days 4, 9, and 14 after the infection, vaginal swabs were taken to estimate Candida burdens. Oral PRC and ITC at 10 mg/kg were both highly effective but not significantly different. At 5 mg/kg, PRC outperformed ITC (P = 0.021) (Fig. 2A). After intravaginal application, superiority of PRC over ITC and MC was significant (Fig. 2B).
FIG. 2.
Comparative efficacy of pramiconazole (PRC) and itraconazole (ITC) after oral (PO) treatment (once daily [s.i.d.]) at 10 and 5 mg/kg (A) and comparison with itraconazole (ITC) and miconazole (MC) results after topical (TP) treatment (twice daily [b.i.d.]) with 1% (wt/wt) cream (B) in the C. albicans vaginitis model in rats. The AUC representing the entire infection burden over the 3 days of sampling is shown on the y axis. The different groups are shown on the x axis. VIC, vehicle-treated control group. *, P = 0.01 to 0.05 (two-tailed t test). ***, P < 0.001 (two-tailed t test).
In conclusion, pramiconazole has potent in vitro anti-dermatophyte and anti-yeast activities comparable to those of current reference drugs. In dermatomycosis and VVC animal models, oral pramiconazole performs better than itraconazole and terbinafine and shows a higher intrinsic in vivo efficacy, as also demonstrated after topical application. Our findings support the potential of pramiconazole as a promising candidate for treatment of topical mycoses.
Acknowledgments
This work was supported by a grant (IOF-SBO) of the Research Council of the University of Antwerp, the Flemish Agency for Innovation by Science and Technology (IWT), and Stiefel, a GSK company.
Footnotes
Published ahead of print on 30 August 2010.
REFERENCES
- 1.Ameen, M. 2010. Epidemiology of superficial fungal infections. Clin. Dermatol. 28:197-201. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., and J. H. Rex. 2007. Antifungal agents, p. 1949-1960. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 3.Ausma, J., G. Pennick, H. Bohets, V. van de Velde, M. Borgers, and A. Fothergill. 2007. Absence of an active metabolite for the triazole antifungal pramiconazole. Acta Derm. Venereol. 87:22-26. [DOI] [PubMed] [Google Scholar]
- 4.Borgers, M., H. Degreef, and G. Cauwenberg. 2005. Fungal infections of the skin: infection process and antimycotic therapy. Curr. Drug Targets 6:849-862. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, H. W., A. H. Groll, C. Chiou, and T. J. Walsh. 2004. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 64:1997-2020. [DOI] [PubMed] [Google Scholar]
- 6.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 7.Cos, P., A. J. Vlietinck, D. V. Berghe, and L. Maes. 2006. Anti-infective potential of natural products: how to develop a stronger in vitro “proof-of-concept.” J. Ethnopharmacol. 106:290-302. [DOI] [PubMed] [Google Scholar]
- 8.Decroix, J., J. Ausma, G. Cauwenbergh, M. Borgers, and L. Wouters. 2008. The efficacy of oral treatment with pramiconazole in tinea pedis and tinea cruris/corporis: two exploratory phase IIa trials. Br. J. Dermatol. 158:854-856. [DOI] [PubMed] [Google Scholar]
- 9.Donders, G., J. Ausma, L. Wouters, G. Cauwenbergh, M. Borgers, and D. Janssens. 2008. Efficacy of a single oral dose of 200 mg pramiconazole in vulvovaginal yeast infections: an exploratory phase IIa trial. Acta Derm. Venereol. 88:462-466. [DOI] [PubMed] [Google Scholar]
- 10.Faergemann, J., J. Ausma, and M. Borgers. 2006. In vitro activity of R126638 and ketoconazole against Malassezia species. Acta Derm. Venereol. 86:1-4. [DOI] [PubMed] [Google Scholar]
- 11.Faergemann, J., J. Ausma, L. Vandenplassche, and M. Borgers. 2007. The efficacy of oral treatment with pramiconazole in pityriasis versicolor: a phase IIa trial. Br. J. Dermatol. 156:1385-1387. [DOI] [PubMed] [Google Scholar]
- 12.Geria, A. N., and N. S. Scheinfeld. 2008. Pramiconazole, a triazole compound for the treatment of fungal infections. IDrugs 11:661-670. [PubMed] [Google Scholar]
- 13.Gupta, A. K., and E. A. Cooper. 2008. Update in antifungal therapy of dermatophytosis. Mycopathologia 166:353-367. [DOI] [PubMed] [Google Scholar]
- 14.Hainer, B. L. 2003. Dermatophyte infections. Pract. Ther. 67:101-108. [PubMed] [Google Scholar]
- 15.McCathie, R. 2006. Vaginal discharge: common causes and management. Curr. Obstet. Gynaecol. 16:211-217. [Google Scholar]
- 16.Meerpoel, L., L. J. J. Backx, L. J. E. Van der Veken, J. Heeres, D. Corens, A. De Groot, F. C. Odds, F. Van Gerven, F. A. A. Woestenborghs, A. Van Breda, M. Oris, P. Van Dorsselaer, G. H. M. Willemsens, K. J. P. Vermuyten, P. J. M. G. Marichal, H. F. Vanden Bossche, J. Ausma, and M. Borgers. 2005. Synthesis and in vitro and in vivo structure-activity relationships of novel antifungal triazoles for dermatology. J. Med. Chem. 48:2184-2193. [DOI] [PubMed] [Google Scholar]
- 17.Odds, F., J. Ausma, F. Van Gerven, F. Woestenborghs, L. Meerpoel, J. Heeres, H. Vanden Bossche, and M. Borger. 2004. In vitro and in vivo activities of the novel azole antifungal agent R126638. Antimicrob. Agents Chemother. 48:388-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petranyi, G., J. G. Meingassner, and H. Mieth. 1987. Activity of terbinafine in experimental fungal infections of laboratory animals. Antimicrob. Agents Chemother. 31:1558-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piérard, G. E., J. Ausma, F. Henry, V. Vroome, L. Wouters, M. Borgers, G. Cauwenbergh, and C. Piérard-Franchimont. 2007. A pilot study on seborrheic dermatitis using pramiconazole as a potent oral anti-Malassezia agent. Dermatology 214:162-169. [DOI] [PubMed] [Google Scholar]
- 20.Ryder, N. S., and B. Favre, 1997. Antifungal activity and mechanism of action of terbinafine. Rev. Contemp. Pharmacother. 8:275-287. [Google Scholar]
- 21.Sobel, J. D. 2007. Vulvovaginal candidosis. Lancet 369:1961-1971. [DOI] [PubMed] [Google Scholar]
- 22.Sobue, S., K. Sekiguchi, and T. Nabeshima. 2004. Intracutaneous distributions of fluconazole, itraconazole, and griseofulvin in guinea pigs and binding to human stratum corneum. Antimicrob. Agents Chemother. 48:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiballi, R. N., X. He, L. T. Zarins, S. G. Revankar, and C. A. Kauffman. 1995. Use of a colorimetric system for yeast susceptibility testing. J. Clin. Microbiol. 33:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treiber, A., W. Pitterman, and H. Schuppe. 2001. Efficacy testing of antimycotic prophylactics in an animal model. Int. J. Hyg. Environ. Health 204:1-5. [DOI] [PubMed] [Google Scholar]