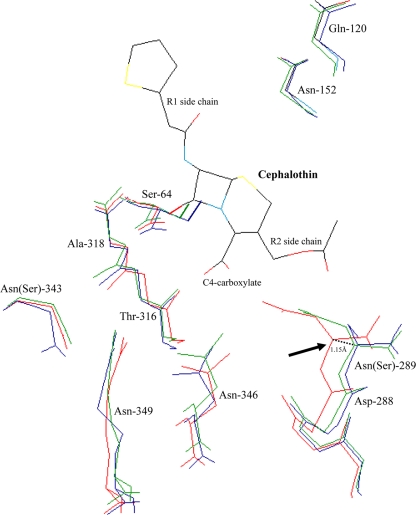
FIG. 1.
Superimposition of the crystallographic structures of CMY-2 β-lactamase (green; PDB code 1ZC2), ACT-1 enzyme (blue; PDB code 2ZC7) 31, and the AmpC β-lactamase of E. coli K-12 (red; PDB code 1KVL). Amino acids that are involved in the substrate binding (Gln-120, Asn-152, Ser-287, Asp-288, Ser-289, Thr-316, Asn-346, and Arg-349) and that constitute the oxyanion binding pocket (Ser-64 and Ser-318) are represented (2, 16, 28). The lateral side chains of Asn-289, Asn-346, and Arg-349, which contribute to the C4-carboxylate β-lactam binding (2, 16, 28), are shown. The side chain of the reactive Ser-64, which attacks the carbonyl carbon of the β-lactam ring, is shown in boldface.