Abstract
Growth of Bacillus subtilis cells, normally adapted at Earth-normal atmospheric pressure (∼101.3 kPa), was progressively inhibited by lowering of pressure in liquid LB medium until growth essentially ceased at 2.5 kPa. Growth inhibition was immediately reversible upon return to 101.3 kPa, albeit at a slower rate. A population of B. subtilis cells was cultivated at the near-inhibitory pressure of 5 kPa for 1,000 generations, where a stepwise increase in growth was observed, as measured by the turbidity of 24-h cultures. An isolate from the 1,000-generation population was obtained that showed an increase in fitness at 5 kPa when compared to the ancestral strain or a strain obtained from a parallel population that evolved for 1,000 generations at 101.3 kPa. The results from this preliminary study have implications for understanding the ability of terrestrial microbes to grow in low-pressure environments such as Mars.
Microorganisms grow optimally within a characteristic range of fundamental physical parameters such as temperature, osmolarity, pH, and pressure. Cells that can grow at the extreme limits of these parameters are known collectively as extremophiles (reviewed in reference 8). Thus, in extreme environments on Earth there exist halophiles in hypersaline niches, psychrophiles and thermophiles in extreme cold and hot environments, and acidophiles or alkaliphiles in environments of extreme acidic or basic pH. Regarding extremes of pressure, piezophiles (barophiles) have been isolated from high-pressure (hyperbaric) submarine environments and have been studied rather extensively; in addition, high pressure has been shown to exert lethal and inhibitory effects on various microbial systems not normally adapted to high pressure (reviewed in reference 19). On the opposite extreme, there has been very little investigation concerning the survival and growth of microbes under conditions of extremely low atmospheric pressure (hypobaria), primarily because such environments do not exist in nature on the Earth's surface. However, some investigators in the emerging field of astrobiology have become concerned with microbial survival and/or growth at low pressures because (i) the closest potential life-bearing planet, Mars, contains a low-pressure atmosphere, and (ii) the possibility exists that terrestrial microbes could be transferred from Earth to Mars as a result of natural impacts (23) or human spaceflight activities (24). Most of the experiments conducted have concentrated on testing the ability of terrestrial microbes merely to survive in the Mars surface environment (reviewed in references 6, 23, and 28); relatively few experiments have tested the ability of specific Earth microbes to grow or metabolize at reduced pressure (12, 14). We previously reported that growth of at least 37 different microorganisms on semisolid agar medium was inhibited at pressures approaching ∼2.5 to 3.5 kPa (3, 4, 27, 30). (Note that atmospheric pressures at the surfaces of Earth and Mars average ∼101.3 kPa and ∼0.7 kPa, respectively [26].) These observations suggest that there exists a low-pressure barrier to the growth of terrestrial microbes. We reasoned that one experimental approach to studying the cellular target(s) and molecular mechanism(s) involved in the prokaryotic response to hypobaric stress would be to use reduced pressure as a selective condition to isolate and characterize mutant strains that had evolved an enhanced capability for growth at low pressure; the first results of these experiments are presented in this communication.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The ancestral strain used in this study was our common laboratory stock of Bacillus subtilis, strain 168. Congenic B. subtilis strains WN624 (trpC2 amyE::spc) and WN628 (trpC2 amyE::cat) were derived from strain 168 by transformation (17). Luria-Bertani (LB) medium (21) was used throughout and was supplemented with spectinomycin (Spc) (100 μg/ml) or chloramphenicol (Cm) (5 μg/ml) to select for WN624 or WN628, respectively, and their descendants. For growth under Earth-normal conditions, liquid cultures were incubated in a water bath with moderate rotary shaking (∼150 rpm). For growth with limited oxygen, liquid cultures were incubated in filled screw-cap test tubes placed vertically in an incubator without shaking. Where indicated, the alternate electron acceptors sodium nitrate (11) and sodium sulfate (1) were added to LB to final concentrations of 10 mM and 1 mM, respectively. For growth at low pressure, cultures in Klett flasks were grown under vacuum in a water bath with moderate rotary shaking (∼150 rpm). Vacuum was supplied by a pumping system (KNF Neuberger, Trenton, NJ) fitted with 0.2-μm-pore in-line air filters. Growth was measured at Earth-normal atmospheric pressure (101.3 ± 0.6 kPa) and 10, 7.5, 5, and 2.5 kPa. Optical densities (ODs) were measured using a Klett-Summerson colorimeter fitted with the no. 66 (red, 660-nm) filter. (Note that for purposes of comparison, 100 Klett units = 1 OD unit at 660 nm [OD660].)
Conditions for laboratory evolution of bacteria were essentially as described previously (17, 18) with slight modifications. Briefly, strains WN624 and WN628 were propagated in 125-ml sidearm (Klett) flasks in 10 ml of liquid LB medium containing the appropriate selective antibiotic in a rotary shaker bath at 27°C. Strain WN628 was propagated at 101.3 ± 0.6 kPa, while strain WN624 was propagated at 5.0 ± 0.2 kPa. At daily intervals, the OD of each population was determined, each culture was diluted 1:100 into fresh selective medium, and incubation continued. Under this regimen, each population progressed through ∼6.6 generations per day. At ∼50-generation intervals, an aliquot of each culture was stored in 25% (vol/vol) glycerol at −70°C.
Competition experiments.
Strains to be compared in pairwise combination were cultivated overnight in selective LB liquid at 27°C and normal atmospheric pressure. Fifty microliters of two different overnight cultures, containing approximately equal numbers of cells, was inoculated into 10 ml of nonselective LB medium in a 125-ml Erlenmeyer flask, cultivated at 101.3 kPa or 5 kPa, and diluted 1:100 into fresh LB each day, as described above. At daily intervals, an aliquot was removed from each culture and serial 10-fold dilutions in phosphate-buffered saline (PBS) buffer (25) were plated on both plates containing LB medium plus Cm (LB+Cm) and LB medium plus Spc (LB+Spc). The proportion of the total population consisting of chloramphenicol-resistant (Cmr) or spectinomycin-resistant (Spcr) colonies was calculated for each mixed population and plotted versus elapsed generations.
Statistical analyses.
Basic statistical parameters and analyses of variance (ANOVA) were performed using commercial statistical software (Kaleidagraph, version 3.6.2; Synergy Software, Reading, PA). Differences with P values of ≤0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Growth inhibition of B. subtilis at low pressure.
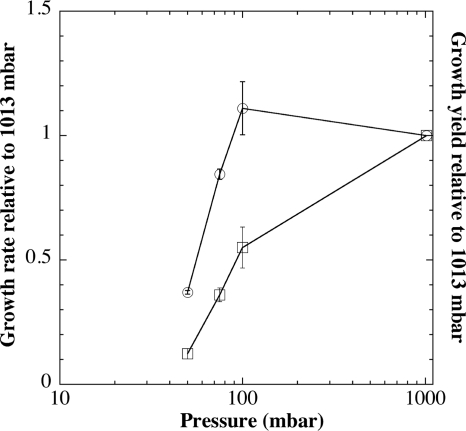
Previous results showed that incubation of several different microorganisms on agar plates at progressively lower pressures resulted in a diminution and eventual cessation of growth (4, 27, 30). To investigate this effect further, B. subtilis strain 168 was cultivated at 27°C in liquid LB medium at 101.3, 10, 7.5, 5, and 2.5 kPa. OD measurements were taken to determine (i) the growth rate and (ii) the final 24-h culture yield at each pressure. It was observed that growth rate was unaffected at 10 kPa but decreased semilogarithmically with lowering of pressure to 7.5 and 5 kPa (Fig. 1). Cell yield at 24 h showed a 2-fold decrease at 10 kPa and then a further semilogarithmic decrease with lowering of pressure to 7.5 and 5 kPa (Fig. 1). Growth was essentially undetectable at 2.5 kPa, in agreement with previous results on semisolid medium (30).
FIG. 1.
Growth rate (doublings/hour; circles), and culture yield (Klett units at 24 h; squares) as a function of pressure. B. subtilis 168 was grown in liquid LB at the pressures indicated (101.3, 10, 7.5, and 5 kPa [i.e., 1,013, 100, 75, and 50 mbar, respectively), and the results were normalized to those from strain 168 grown at atmospheric pressure (101.3 kPa), which grew to a final OD660 of 334 ± 2 Klett units with a doubling time of 85 ± 8 min. Data are presented as averages ± standard deviations (n = 3).
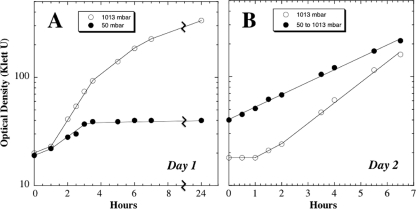
Growth inhibition at 5 kPa was investigated in further detail. Strain 168 was cultivated in LB liquid medium at 101.3 or 5 kPa, and growth was monitored as described above. At 101.3 kPa, a typical growth curve was observed, featuring an ∼1-h lag phase, rapid exponential growth, a gradual entrance into stationary phase, and overnight growth to a high cell density of ∼335 Klett units (Fig. 2A). As expected, the same pattern was repeated upon dilution of the culture into fresh LB medium at 101.3 kPa on the following day (Fig. 2B). In contrast, when strain 168 was grown at 5 kPa, the cells also experienced an ∼1-h lag period, but exponential growth proceeded at a lower rate and the cells abruptly entered stationary phase at a low cell density of ∼40 Klett units; this low density persisted for 24 h (Fig. 2A). Upon return of the culture from 5 kPa to 101.3 kPa on the following day, the cells resumed exponential growth immediately, without any discernible lag phase, albeit at a slower growth rate than cells precultivated at 101.3 kPa (Fig. 2B). Although the molecular basis for low-pressure growth inhibition is presently unknown, the data suggest two important features. First, the immediate resumption of vegetative growth upon return to atmospheric pressure indicates the existence of some target molecule(s) within the cells that can be reversibly inactivated by pressure changes without being resynthesized de novo. Second, it appeared that cells grown for 24 h at 5 kPa had undergone a physiological alteration slowing their subsequent growth upon return to 101.3 kPa; furthermore, this alteration persisted for at least 2 doublings after return to 101.3 kPa (Fig. 2).
FIG. 2.
(A) Day 1. Growth of B. subtilis strain 168 cultivated at 27°C in LB medium at 101.3 kPa (1,013 mbar) (open circles) or 5 kPa (50 mbar) (filled circles). (B) Day 2. Strain 168 grown at 101.3 kPa was diluted 1:20 into fresh LB medium and cultivated at 101.3 kPa, 27°C (open circles). The flask containing strain 168 grown at 5 kPa was simply repressurized to 101.3 kPa and cultivated at 27°C (filled circles).
Evolution of B. subtilis to enhanced growth at low pressure.
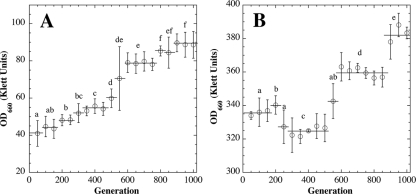
We reasoned that growth inhibition at 5 kPa could be used as an environmental stress to select for enhanced growth at low pressure and furthermore that analysis of low-pressure-evolved stains could yield insights into the molecular mechanisms underlying growth at low pressure. Therefore, the following experiment was performed. Congenic derivatives of B. subtilis strain 168, strains WN624 (Spcr) and WN628 (Cmr), were subjected to laboratory evolution at 5 kPa and 101.3 kPa, respectively, for 1,000 generations. Over the 1,000-generation course of the experiment, a progressive increase in 24-h culture OD was observed in both cultures (Fig. 3A and B). In both cultures, the increase was observed to occur in a stepwise manner: i.e., cells went through periods of no significant change in OD, interspersed by periods characterized by increases in OD (Fig. 3). We have previously observed this pattern of “punctuated equilibrium” (reviewed in reference 10) in evolving B. subtilis cultures (17, 18) and interpreted the phenomenon as successive population sweeps by spontaneously arising mutants capable of increased growth under continued selective pressure.
FIG. 3.
(A) Evolution of strain WN624 to enhanced growth at 5 kPa. (B) Evolution of strain WN628 to enhanced growth at atmospheric pressure (∼101.3 kPa). In both panels, the optical density (Klett units) of 24-h cultures was monitored. Data points represent the averages and standard deviations of daily culture OD660 values grouped at 1-week (∼50-generation) intervals. Data points linked by horizontal lines beneath lowercase letters indicate groups of data that were not significantly different by ANOVA (P > 0.05; n = 7).
Fitness measurements by competition experiments.
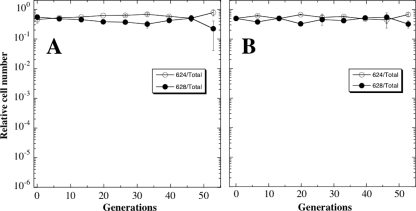
Two single-colony isolates were streak purified—one from each culture grown at normal atmospheric pressure (101.3 kPa) and at 5 kPa—and were designated strains WN1105 (Cmr) and WN1106 (Spcr), respectively. In order to assess the relative fitness of the evolved strains versus their ancestors and each other, pairwise competition experiments were performed. First, ancestral strains WN624 and WN628 were inoculated at equal cell numbers and competed in LB medium at either 101.3 kPa (Fig. 4A) or 5 kPa (Fig. 4B) for ∼50 generations. Neither ancestral strain demonstrated a selective advantage over the other at either pressure, thus ensuring that the amyE::spc or amyE::cat markers in the otherwise identical strains were selectively neutral. It was important to establish that ancestral strains WN624 and WN628 showed the same relative fitness, because each ancestor was subsequently competed against the evolved strain from the other population, to take advantage of the difference in their antibiotic resistance markers.
FIG. 4.
Competition experiments between ancestral strain WN624 (open circles) and WN628 (filled circles) propagated together in LB medium without antibiotics at 101.3 kPa (A) or 5 kPa (B). Data are averages ± standard deviations from duplicate experiments.
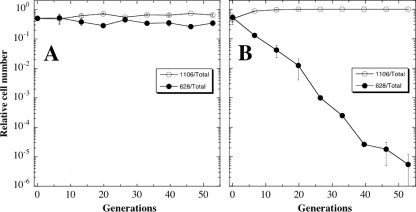
In the next set of competition experiments, ancestral strain WN628 (Cmr) was competed against low-pressure-evolved strain WN1106 (Spcr) (Fig. 5). When the two strains were propagated together at 101.3 kPa, no selective advantage was observed (Fig. 5A). However, when the two strains were cocultivated at 5 kPa, strain WN1106 showed a clear growth advantage at low pressure, and ancestral strain WN628 was lost from the mixed population at a rate of 1 log per ∼10 generations (Fig. 5B). Thus, after 1,000 generations of evolution at 5 kPa, strain WN1106 indeed appeared to have gained fitness for growth at low pressure.
FIG. 5.
Competition experiments between ancestral strain WN628 (filled circles) and WN1106 (open circles) propagated together in LB medium without antibiotics at 101.3 kPa (A) or 5 kPa (B). Data are averages ± standard deviations from duplicate experiments.
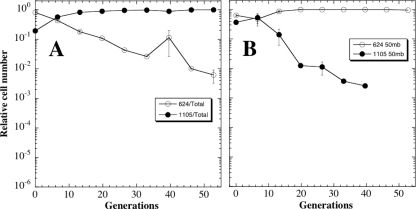
In the next set of experiments, ancestral strain WN624 (Spcr) was competed against strain WN1105 (Cmr), which had evolved for 1,000 generations at 101.3 kPa. Improved growth of WN1105 in LB at atmospheric pressure likely resulted from improved utilization of the residual nutrients in stationary phase, as was observed in previous long-term evolution experiments (17). As expected, when the competition experiment was performed at 101.3 kPa, strain WN1105 exhibited increased fitness over the ancestor strain WN624, which was lost from the mixed population at a rate of 1 log per ∼28 generations (Fig. 6A). However, when the same competition experiment was performed at 5 kPa, the evolved strain WN1105 showed a distinct decrease in fitness compared to ancestral strain WN624, and strain WN1105 was lost from the mixed population at a rate of 1 log per ∼16 generations (Fig. 6B). These results are in sharp contrast to those observed in the competition between strains WN1106 and WN628 at normal atmospheric pressure, where no distinct difference in fitness was observed (Fig. 5A). The results suggested that prolonged evolution under constant conditions (1,000 generations, LB medium, 27°C, 101.3 kPa) may have resulted in a reduction in the ability of strain WN1105 to adjust to a change in its environmental atmospheric pressure. In previous long-term evolution experiments, we also observed a loss of phenotypic plasticity during long-term propagation under constant conditions; transcription microarray experiments indicated that loss of phenotypic plasticity could be attributed to a global loss of transcriptome flexibility in response to environmental changes (16).
FIG. 6.
Competition experiments between ancestral strain WN624 (filled circles) and WN1105 (open circles) propagated together in LB medium without antibiotics at 101.3 kPa (1,013 mbar) (A) or 5 kPa (50 mbar) (B). Data are averages ± standard deviations from duplicate experiments.
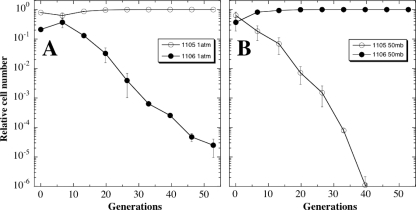
Finally, both evolved strains WN1106 (Spcr) and WN1105 (Cmr) were competed against one another at 101.3 kPa or at 5 kPa (Fig. 7). The results obtained showed that each strain had become better adapted for growth at the pressure for which it had been evolved. At 101.3 kPa, strain WN1106 was preferentially lost from the mixed culture at a rate of 1 log per ∼11 generations (Fig. 7A), and when cocultivated at 5 kPa, strain WN1105 was lost from the mixed culture at a rate of 1 log per ∼7 generations (Fig. 7B).
FIG. 7.
Competition experiments between 5.0-kPa-evolved strain WN1106 (filled circles) and 101.3-kPa-evolved strain WN1105 (open circles) propagated together in LB medium without antibiotics at 101.3 kPa (1,101 mbar) (A) or 5 kPa (50 mbar) (B). Data are averages ± standard deviations from duplicate experiments.
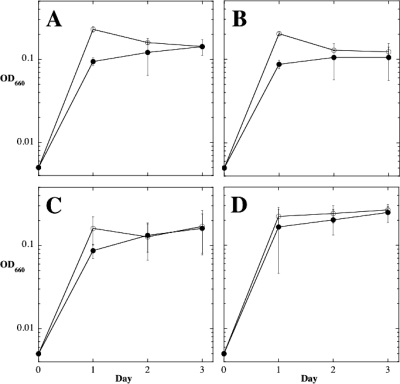
The results presented in this preliminary study demonstrate that low pressure inhibits both the growth rate and final growth yield of B. subtilis cells. What could cause inhibition of bacterial cell growth at low pressure, and how might strain WN1106 have evolved to overcome this inhibition? One obvious possibility immediately presents itself: low-pressure growth inhibition may result simply from oxygen starvation. In our experiments, lowering of pressure in the headspace above the culture from ∼101.3 kPa to 5 kPa would lead to a corresponding ∼20-fold decrease in the partial pressure of dissolved gases (notably oxygen) in the culture. B. subtilis is capable of growth under oxygen limitation via fermentation or anaerobic respiration (reviewed in reference 22), but LB medium is a poor source of either fermentable carbon sources or alternative electron acceptors. Therefore, both the growth rate and final cell yield of B. subtilis in LB medium would be expected to be lower at the reduced dissolved oxygen concentrations resulting from low pressure. Considering this possible scenario, immediate resumption of growth of wild-type strain 168 by transition from 5 kPa to ∼101.3 kPa (Fig. 2) may merely result from the return of sufficient oxygen to an oxygen-starved culture, and, furthermore, strain WN1106 may have evolved an ability for enhanced low-oxygen growth, rather than low-pressure growth per se. In order to test this notion, we cultivated ancestral strain WN624 and low-pressure-evolved strain WN1106 in liquid LB medium under oxygen-limited conditions and compared their growth profiles (Fig. 8). Strain WN1106 was clearly not more capable of anaerobic growth than strain WN624 in LB medium (Fig. 8A). Nor was strain WN1106 capable of enhanced growth under oxygen limitation when cultivated in LB medium containing either of the alternate electron acceptors sulfate (Fig. 8B) or nitrate (Fig. 8C) or both sulfate and nitrate (Fig. 8D). Thus, the evidence strongly suggests that strain WN1106 has not evolved simply to enhanced growth under oxygen-limited conditions.
FIG. 8.
Growth of strains WN624 (open circles) and WN1106 (filled circles) under conditions of oxygen limitation. Cultures were grown in liquid LB medium with no addition (A), 1 mM sodium sulfate (B), 10 mM sodium nitrate (C), and both 1 mM sodium sulfate and 10 mM sodium nitrate (D). Data are averages ± standard deviations from duplicate experiments.
For insights into other possible reasons for low-pressure growth inhibition and the evolution of enhanced growth of strain WN1106 at low pressure, it may be instructive to turn to our current understanding of life at the opposite extreme (i.e., high pressure). From the study of high-pressure microbiology, it has been shown that pressure changes can exert effects on the synthesis and activity of numerous cellular targets, including lipids, enzymes, and nucleic acids (2). For example, the ability of deep-sea piezophiles to grow at extreme high pressures has been correlated with the presence in their membranes of a large percentage of mono- and polyunsaturated fatty acids (13). Piezophiles can vary the ratio of unsaturated-to-saturated fatty acids in their membranes in a direct relationship with pressure (7), in order to maintain a homeostatic membrane fluidity and consequently the activity of various membrane-associated proteins such as transporters, respiratory enzymes, or environmental sensors (13). In addition, pressure-related changes to the stability and activity of various cytosolic enzymes such as RNA polymerase have also been documented (13). Interestingly, low-pressure growth inhibition of B. subtilis 168 appeared to be immediately reversible upon return to normal atmospheric pressure, but at a lower rate (Fig. 2). This observation suggests that inhibition may be caused by in part by reversible inactivation of some critical cell components not requiring de novo synthesis and in part by affecting some cell components that do require recovery time. In this scenario, strain WN1106 may harbor mutations rendering such targets less sensitive to inactivation by low pressure or regulatory mutations enhancing the ability of cells to cope with low-pressure stress.
Results from our preliminary studies demonstrate for the first time that microbial evolution is possible at low pressure, yielding descendants that are adapted for enhanced growth at low pressures. How does this finding impact the field of astrobiology? A key unanswered question for the robotic and human exploration of Mars is whether terrestrial microorganisms can adapt to and acquire the capability for active metabolism and replication at, the ∼0.7-kPa atmospheric pressure at the martian surface. Diverse microbial communities including extremophiles and sporeformers have been recovered from spacecraft surfaces and processing facilities prior to launch of spacecraft to Mars (9, 15, 31), and the survival of some of these species during the Earth-to-Mars interplanetary transit phase is quite probable (23, 24, 26). However, upon landing on Mars, at least 13 separate biocidal factors in addition to low pressure are likely to be present on the martian surface (29, 30). At present it is unclear whether terrestrial microorganisms can overcome the entire collection of interacting biocidal factors in order to evolve the capability for replication on Mars. Results from future research probing the lower limits of replication and evolution under hypobaric conditions will directly constrain planetary protection protocols for spacecraft and the search for an extant martain microbiota (24) and discover whether terrestrial microorganisms will be able to proliferate on the surface or near subsurface of Mars (23).
Probing the low-pressure limit for life has fundamental implications beyond the astrobiology of low-pressure environments such as Mars. In the experiments described in this communication, we imposed a 20-fold reduction in pressure on B. subtilis, a bacterium normally adapted to Earth surface atmospheric pressure—from 101.3 kPa to 5 kPa. This pressure change could be considered analogous to the hypothetical removal of a deep-sea piezophile from the Marianas Trench (10,911 m below sea level at ∼110 MPa) to a depth of only 540 m (∼5 MPa). It has previously been demonstrated that the growth rate of piezophiles is dramatically slowed at progressively lower pressures (5, 20), in a manner analogous to that seen for B. subtilis in Fig. 1. Therefore, it is possible that similar mechanisms are at work in both cases and that exploring the low-pressure limit of microorganisms normally adapted to growth at the Earth's surface might provide insights into the factors limiting growth of piezophiles at the lower limits of their own pressure ranges. In order to further understand the genetic response of B. subtilis to low-pressure stress, current experimental efforts are being concentrated on using transcription microarrays to identify genes whose transcription is altered in (i) the ancestral versus low-pressure-evolved strains, (ii) in cells propagated at 101.3 kPa versus 5 kPa, and (iii) in microaerophilic environments induced by low pressure versus those induced by oxygen limitation at atmospheric pressure.
Acknowledgments
We thank the anonymous reviewers for their insightful comments.
This work was supported by a grant from the NASA Exobiology and Evolutionary Biology Program (NNX08AO15G).
Footnotes
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Auger, S., A. Danchin, and I. Martin-Verstraete. 2002. Global expression profile of Bacillus subtilis grown in the presence of sulfate or methionine. J. Bacteriol. 184:5179-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, D. H., G. Ferguson, and G. Valle. 2008. Adaptations of the psychrotoloerant piezophile Photobacterium profundum strain SS9, p. 319-337. In C. Michiels, D. Bartlett, and A. Aertsen (ed.), High-pressure microbiology. ASM Press, Washington, DC.
- 3.Berry, B., D. G. Jenkins, and A. C. Schuerger. 2010. Effects of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquifaciens. Appl. Environ. Microbiol. 76:2377-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, B., A. C. Schuerger, and W. L. Nicholson. 2006. Proliferation of common spacecraft contaminants in simulated martian conditions is limited. Astrobiology 6:254. [Google Scholar]
- 5.Boonyaratanakornkit, B. B., and D. S. Clark. 2008. Physiology and biochemistry of Methanocaldococcus jannaschi at elevated pressures, p. 293-304. In C. Michiels, D. Bartlett, and A. Aertsen (ed.), High-pressure microbiology. ASM Press, Washington, DC.
- 6.Fajardo-Cavazos, P., A. C. Schuerger, and W. L. Nicholson. 2007. Testing interplanetary transfer of bacteria by natural impact phenomena and human spaceflight activities. Acta Astronaut. 60:534-540. [Google Scholar]
- 7.Fang, J., and D. A. Bazylinski. 2008. Deep sea geomicrobiology, p. 237-264. In C. Michiels, D. Bartlett, and A. Aertsen (ed.), High-pressure microbiology. ASM Press, Washington, DC.
- 8.Gerday, C., and N. Glansdorff (ed.). 2007. Physiology and biochemistry of extremophiles. ASM Press, Washington, DC.
- 9.Ghosh, S., S. Osman, P. Vaishampayan, and K. Venkateswaran. 2010. Recurrent isolation of extremotolerant bacteria from the clean room where Phoenix spacecraft components were assembled. Astrobiology 10:325-335. [DOI] [PubMed] [Google Scholar]
- 10.Gould, S. J. 2007. Punctuated equilibrium. Belknap Press/Harvard University Press, Cambridge, MA.
- 11.Hoffmann, T., N. Frankenberg, M. Marino, and D. Jahn. 1998. Ammonification in Bacillus subtilis utilizing dissimilatory nitrite reductase is dependent on resDE. J. Bacteriol. 180:186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanervo, E., K. Lehto, K. Ståhle, J. Lehto, and P. Mäenpää. 2005. Characterization of growth and photosynthesis of Synechocystis sp. PCC6803 cultures under reduced atmospheric pressures and enhanced CO2 levels. Int. J. Astrobiol. 4:979-1000. [Google Scholar]
- 13.Kato, C., Y. Nogi, and S. Arakawa. 2008. Isolation, cultivation, and diversity of deep-sea piezophiles, p. 203-217. In C. Michiels, D. Bartlett, and A. Aertsen (ed.), High-pressure microbiology. ASM Press, Washington, DC.
- 14.Kral, T. A., T. S. Altheide, A.E. Lueders, T. H. Goodhart, B. T. Virden, W. Birch, K. L. Howe, and P. Gavin. 2010. Methanogens: a model for life on Mars, abstr. 5084. Abstr. 2010 Astrobiol. Sci. Conf. (AbSciCon 2010), Houston, TX.
- 15.La Duc, M. T., R. Kern, and K. Venkateswaran. 2004. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47:150-158. [DOI] [PubMed] [Google Scholar]
- 16.Maughan, H., C. W. Birky, Jr., and W. L. Nicholson. 2009. Transcriptome divergence and the loss of plasticity in Bacillus subtilis after 6,000 generations of evolution under relaxed selection for sporulation. J. Bacteriol. 191:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maughan, H., V. Callicotte, A. Hancock, C. W. Birky, Jr., W. L. Nicholson, and J. Masel. 2006. The population genetics of trait deterioration in experimental populations of Bacillus subtilis. Evolution 60:686-695. [PubMed] [Google Scholar]
- 18.Maughan, H., J. Masel, C. W. Birky, Jr., and W. L. Nicholson. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michiels, C., D. Bartlett, and A. Aertsen (ed.). 2008. High-pressure microbiology. ASM Press, Washington, DC.
- 20.Miller, J. F., N. N. Shah, C.M. Nelson, J. M. Ludlow, and D. S. Clark. 1988. Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl. Environ. Microbiol. 54:3039-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, J. M. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Nakano, M. M., and P. Zuber. 2002. Anaerobiosis, p. 393-404. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 23.Nicholson, W. L. 2009. Ancient micronauts: interplanetary transport of endolithic microbes by cosmic impacts. Trends Microbiol. 17:243-250. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson, W. L., A. C. Schuerger, and M. S. Race. 2009. Migrating microbes and planetary protection. Trends Microbiol. 17:389-392. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. J. Wiley and Sons, Chichester, United Kingdom.
- 26.Schuerger, A. C. 2004. Microbial ecology of the surface exploration of Mars with human-operated vehicles, p. 363-386. In C. S. Cockell (ed.), Martian expedition planning. American Astronautical Society publication AAS 03-322. Univelt Publishers, Santa Barbara, CA.
- 27.Schuerger, A. C., B. Berry, and W. L. Nicholson. 2006. Terrestrial bacteria typically recovered from Mars spacecraft do not appear able to grow under simulated martian conditions, abstr. 1397. Abstr. 37th Lunar Planetary Sci. Conf., Houston, TX, 13 to 17 March 2006.
- 28.Schuerger, A. C., R. L. Mancinelli, R. G. Kern, L. J. Rothschild, and C. P. McKay. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus 165:253-276. [DOI] [PubMed] [Google Scholar]
- 29.Schuerger, A. C., D. W. Ming, and D. C. Golden. 2010. Biotoxcity of Mars analog soils: microbial dispersal into desiccated soils versus emplacement in salt or ice inclusions, abstr. 5336. Abstr. 2010 Astrobiol. Sci. Conf. (AbSciCon 2010), Houston, TX.
- 30.Schuerger, A. C., and W. L. Nicholson. 2006. Interactive effects of hypobaria, low temperature, and CO2 atmosphere inhibit the growth of mesophilic Bacillus spp. under simulated martian conditions. Icarus 185:143-152. [Google Scholar]
- 31.Venkateswaran, K., M. Satomi, S. Chung, R. Kern, R. Koukol, C. Basic, and D. White. 2001. Molecular microbial diversity of a spacecraft assembly facility. Syst. Appl. Microbiol. 24:311-320. [DOI] [PubMed] [Google Scholar]