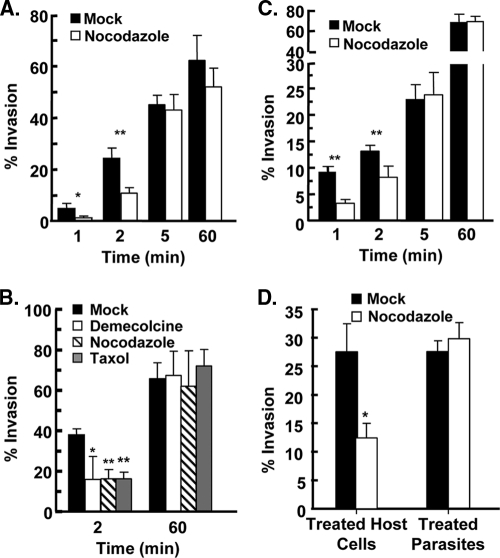
Fig. 2.
Host microtubules are important for Toxoplasma Invasion. (A) HFFs were treated with dimethyl sulfoxide (DMSO) or 1 μM nocodazole for 3 h at 37°C. GFP+ parasites were then added to the cells in vehicle- or drug-containing high-K+ buffer and incubated for 20 min. K+ buffer was removed and replaced with DMSO- or nocodazole-containing invasion buffer. At the indicated times, cells were fixed, and the numbers of intracellular and extracellular parasites were determined by differential SAG1 staining. The averages and standard deviations of three independent experiments counting a minimum of 200 randomly selected parasites for each sample are shown. *, P < 0.05; **, P < 0.005 (Student t test). (B) HFFs were mock treated or treated with a 1 μM concentration of the indicated drug for 3 h at 37°C and then infected with GFP+ parasites using the potassium synchronization method. At the indicated times, the cells were fixed and stained with anti-SAG1 to discriminate between extracellular and intracellular parasites. The averages and standard deviations of three independent experiments counting a minimum of 200 randomly selected parasites for each sample are shown (*, P < 0.05; **, P < 0.005 [Student t test]). (C) DMSO- or nocodazole-treated HFFs were infected with GFP+ parasites using the temperature-shift synchronization method. At the indicated times, cells were fixed and stained with anti-SAG1 to discriminate between extracellular and intracellular parasites. The averages and standard deviations of three independent experiments counting a minimum of 200 randomly selected parasites for each sample are shown (**, P < 0.005 [Student t test]). (D) “Treated HFFs” are host cells pretreated with 1 μM nocodazole or DMSO for 3 h at 37°C and then infected with GFP+ parasites. “Treated parasites” are extracellular GFP+ parasites treated with 1 μM nocodazole or DMSO in DMEM for 1 h at 37°C and then washed. The washed parasites were added to host cells in high-K+ buffer, and then invasion buffer was added. Two minutes later, the cells were fixed, and the numbers of invaded parasites were determined by SAG1 differential staining. The averages and standard deviations of three independent experiments counting a minimum of 200 randomly selected parasites for each sample are shown (*, P < 0.05 [Student t test]).