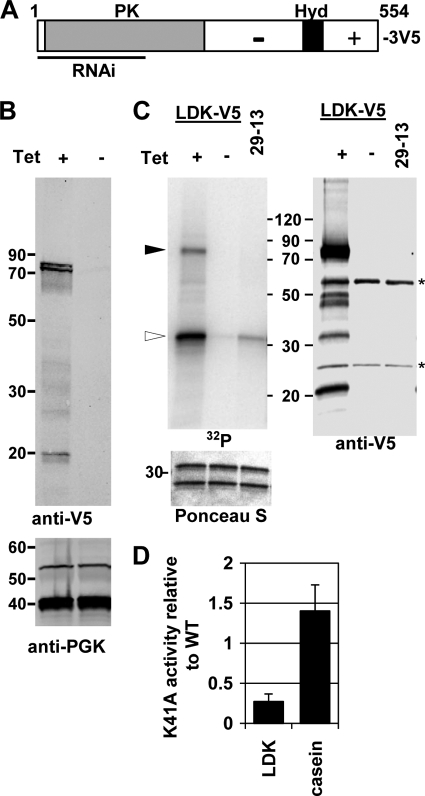
Fig. 1.
Lipid droplet kinase structure and kinase activity. (A) Schematic of LDK, showing location of protein kinase (PK domain) and hydrophobic region (Hyd), which lies in the C-terminal extension. Between the kinase domain and the hydrophobic region, the protein is enriched for acidic aa (−), whereas distal to the hydrophobic region it is enriched for basic aa (+). The region included in the RNAi construct is marked. The annotated sequence is shown in Fig. S1 in the supplemental material. (B) Immunoblot. Lysates were prepared from PF induced (+Tet) or uninduced (−Tet) for LDK-V5 expression. After SDS-PAGE, samples were transferred to nitrocellulose. The blot was incubated with anti-V5 and goat anti-mouse immunoglobulin IRDye800 and scanned. The migration of molecular mass markers (in kilodaltons) is indicated in this and other figures. (C) Kinase assay. Anti-V5 immunoprecipitates from cell expressing (Tet+) or not expressing (Tet−) LDK-V5 were collected and assayed for protein kinase activity in the presence of casein as a candidate exogenous substrate. The gel containing the samples was blotted and subjected to overnight phosphorimaging, as well as Western analysis with anti-V5. Left section (32P), kinase assay. Filled arrowhead, LDK-V5; open arrowhead, upper band of casein. Below is the relevant portion of the same blot stained with Ponceau S to reveal casein. Right section (anti-V5), Western analysis. Asterisks mark the heavy and light chain of the antibodies used for immunoprecipitation, which were detected by the second step antibodies. (D) Phosphotransfer activity of LDK K41A. After immunoprecipitation and kinase assay of wild-type and K41A LDK-V5, 32P associated with the LDK-V5 band and the casein bands was quantitated by phosphorimaging. LDK-V5 was quantitated by immunoblotting of the same gel lanes, allowing normalization of activity. The graph depicts the relative level of phosphorylation by LDK K41A immunoprecipitates compared to the wild type.