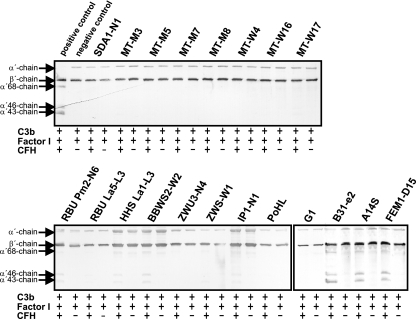
FIG. 4.
Analysis of the functional activities of CFH bound to B. lusitaniae. Spirochetes immobilized to microtiter plates were used to capture CFH. After sequential addition of C3b and factor I, bound CFH retained cofactor activity by enabling factor I-mediated cleavage of C3b to iC3b. Following incubation, the mixture was separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose, and C3b and its degradation products were analyzed using a C3 antiserum (Calbiochem, Darmstadt, Germany). As a positive control, purified CFH (50 ng) was incubated with C3b and factor I, and as a negative control, complement proteins were incubated in the absence of CFH. The mobilities of the α′-chain and the β-chain of C3b and the cleavage products of the α′-chain (α′-68, α′-46, and α′-43) are indicated. +, incubation with all complement proteins; −, incubation in the absence of CFH.