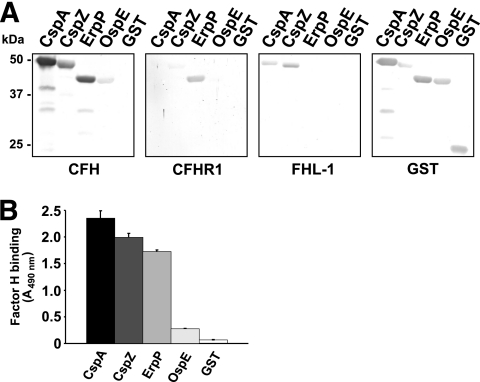
FIG. 6.
Binding capability of OspE of B. lusitaniae MT-M8 to human serum proteins. (A) Binding capabilities of CFH, CFHR1, and FHL1 to purified GST fusion proteins were analyzed by ligand affinity blotting. Recombinant proteins (500 ng/lane) were subjected to 10% Tris-Tricine SDS-PAGE and blotted to nitrocellulose membranes. GST fusion proteins were detected by using anti-goat GST antibody. For detection of CFH and CFHR1 bound to CRASP proteins, membranes were incubated with NHS as a source of CFH or with purified CFHR1. Protein complexes were then visualized using MAb IXF9 or JHD 7.10, respectively. Binding of FHL1 was detected using MAb B22 or polyclonal anti-SCR1-4 antiserum specific for the N-terminal region of CFH and FHL1. The CFH/FHL1-binding CspA and CspZ proteins, the CFH/CFHR1-binding ErpP protein, and purified GST served as controls. (B) Binding of CFH to recombinant CRASP proteins was also quantified by ELISA. Proteins (500 ng each) were immobilized onto a microtiter plate and incubated with purified CFH. For detection of protein complexes, a polyclonal anti-CFH antiserum was used. Reaction mixtures were run in duplicate or triplicate, and all experiments were repeated at least twice, with very similar results; the figure displays a representative experiment. Error bars represent standard deviations (SDs).