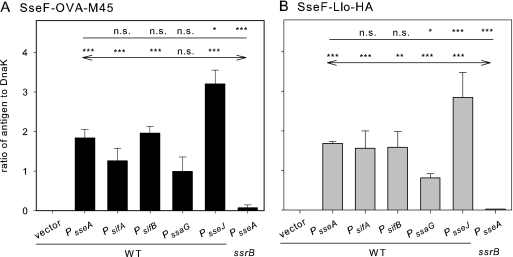
FIG. 3.
Synthesis of fusion proteins with model antigens. The S. enterica serovar Typhimurium wild-type strain with the empty plasmid vector (vector) or harboring plasmids of the expression of sseF::OVA::M45 (A) or sseF::lisA::HA (B) under the control of various SsrAB-controlled promoters was grown in SPI2-inducing minimal media (PCN-P, pH 5.8). As negative controls, ssrB-deficient strain (MvP532, ΔssrB::FRT) harboring plasmids with PsseA-containing expression cassettes was used. Bacteria were harvested after 8 h of culture under inducing conditions, and equal amounts of bacteria (determined based on the optical density at 600 nm) were lysed and subjected to SDS-PAGE and Western blot analyses for the detection of the M45 (A) or HA (B) epitope tag. Blots were probed with fluorescently labeled secondary antibodies, and signal intensities were quantified by using the Odyssey system (Li-Cor). As loading controls, the cytosolic heat shock protein DnaK was detected on the same blot, and signals were quantified. The ratios of the M45 to DnaK or HA to DnaK signals were calculated, and means and standard deviations for three samples are shown. Statistical analyses were performed comparing signals for expression cassettes with various promoters to signals for expression cassettes with PsseA (indicated by plain lines) or by comparing various expression cassettes in a WT background to the PsseA expression cassette in an ssrB background (indicated by lines with arrows). Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.