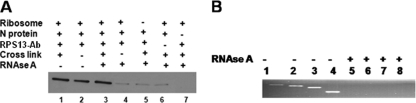
FIG. 1.
(A) Purified 40S subunits were incubated with bacterially expressed and purified N. The ribosome-N mixture was cross-linked with formaldehyde and immunoprecipitated with anti-RPS13 antibody (Ab). Immunoprecipitated material was examined by Western blotting using anti-N antibody (lane 1). The same experiment was performed without cross-linking (lane 2). The experiment performed for lane 3 was same as that for lane 1, except the reaction mixture was treated with RNase A prior to immunoprecipitation. The experiment performed for lane 4 was same as that for lane 3, except that N-40S subunit complexes were not cross-linked. Control immunoprecipitation experiments lacking the ribosome (lane 5) and the anti-RPS13 antibody (lane 6) show the nonspecific binding of N to the beads. However, lane 7 does not show such nonspecific background, due to the lack of N protein in the reaction mixture. (B) To confirm that 18S rRNA was completely digested with RNase A treatment in the samples used in panel A, total RNA was purified from both RNase A-treated and untreated samples and reverse transcribed using random primers. The cDNA was used as the template, and a PCR analysis was performed using four sets of primers targeted to different regions of 18S rRNA. The PCR products generated using primer pairs 5′-ATGCTTGTCTCAAAGATTAAG-3′ and 5′-CGAAAGAGTCCTGTATTGTTA-3′ (lanes 1 and 5), 5′-AGGCCCTGTAATTGGAATGAG-3′ and 5′-TGCGCCGGTCCAAGAATTTCA-3′ (lanes 2 and 6), 5′-AGACGGACCAGAGCGAAAGCA-3′ and 5′-AGCATGCCAGAGTCTCGTTCG-3′ (lanes 3 and 7), and 5′-AACTAGTTACGCGACCCCCGA-3′ and 5′-TAATGATCCTTCCGCAGGTTC-3′ (lanes 4 and 8) are shown.