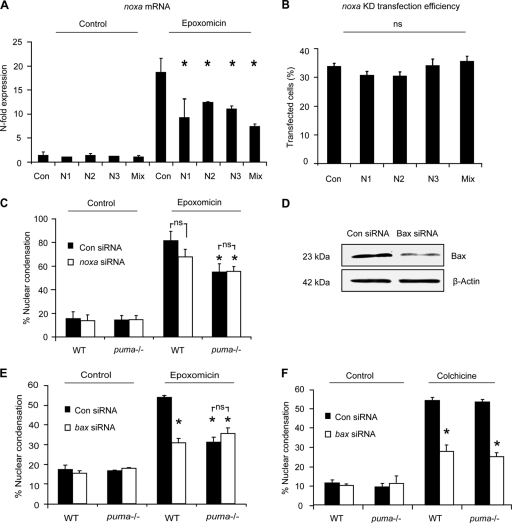
FIG. 4.
Knockdown of proapoptotic Bcl-2 family genes noxa and bax, does not confer additional protection to puma−/− neocortical neurons. Three noxa siRNAs were tested for their ability to attenuate noxa gene expression in neurons. (A) The siRNAs were transfected into neurons during culture preparation (DIV 0) using Amaxa (mouse neofection kit). Neurons were subsequently (DIV 5) treated with epoxomicin for 24 h or vehicle (DMSO, 0.1%), and samples were prepared for RT-qPCR. noxa gene expression was given as n-fold expression over control and normalized to β-actin. (B) The transfection efficiency of Amaxa-transfected neurons at DIV 5 was assessed by quantifying GFP-positive neurons within the cultures. KD, knockdown. (C) The number of apoptotic neurons from GFP-expressing cells in WT and puma−/− in scramble or noxa siRNA-transfected neurons was quantified in control- or epoxomicin (50 nM)-treated samples after 24 h (n = 123 to 150 cells/time point quantified). *, P < 0.05 compared to epoxomicin-treated control siRNA (ANOVA and Tukey's post hoc test). ns, not significant. (D) Western blotting of Bax expression 24 h posttransfection with either control or Bax siRNA sequences. β-Actin served as a loading control. (E) The number of apoptotic nuclei in WT and puma−/− neurons transfected with either Bax siRNA or scramble siRNA was quantified in control- or epoxomicin-treated cultures. siRNA was cotransfected with a plasmid expressing GFP to allow for identification of transfected neurons. (F) WT and puma−/− cortical neurons were transfected with scramble or Bax siRNA and subsequently treated with colchicine (10 μM) or vehicle, and apoptosis was assessed as in panel E. *, P < 0.05 compared drug-treated control siRNA (ANOVA and Tukey's post hoc test). ns, not significant. The experiments were repeated three times with independent culture preparations with similar results.