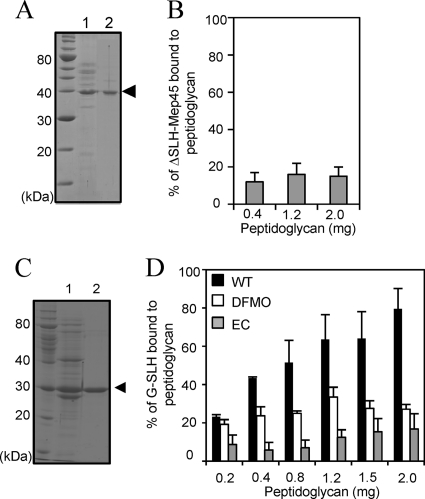
FIG. 3.
Functional analysis of the SLH domain of Mep45. (A) Preparation of Mep45 with the SLH domain truncated (ΔSLH-Mep45). Lane 1, trypsin-digested crude envelopes; lane 2, purified ΔSLH-Mep45 preparation. The arrowhead indicates the ΔSLH-Mep45 bands. (B) Peptidoglycan-binding assay of ΔSLH-Mep45. Solubilized ΔSLH-Mep45 (100 μg/ml) was incubated with 0.4, 1.2, or 2.0 mg of peptidoglycan from wild-type S. ruminantium cells. (C) Preparation of recombinant SLH domain fused with glutathione S-transferase (G-SLH). Lane 1, cell extracts of E. coli BL21(DE3) expressing G-SLH; lane 2, purified G-SLH. The arrowhead indicates the G-SLH band. (D) Peptidoglycan-binding assay of G-SLH of (i) wild-type S. ruminantium cells (WT [closed bars]), (ii) S. ruminantium cells cultured in the presence of 10 mM DFMO (open bars), and (iii) the E. coli lpo mutant (EC [shaded bars]). In panels B and D, anti-glutathione S-transferase antiserum was used to detect G-SLH and relative amounts of peptidoglycan-associated G-SLH were represented as the amount relative to initial amount of G-SLH added to the reaction mixture (mean ± SD of triplicate experiments).