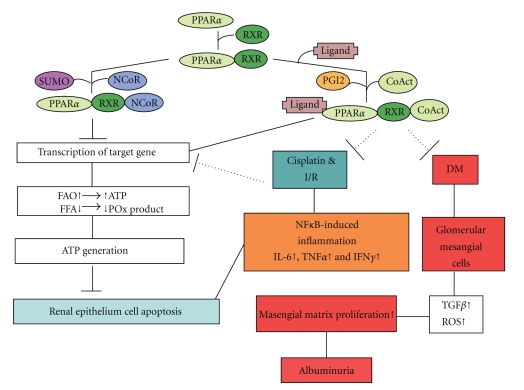
Figure 1.
Schematic diagram presenting the signaling pathways of PPARα involved in the mechanisms of ischemic/reperfusion-, drugs-, or diabetic-induced renal damage. PPARα forms heterodimer with RXR. In the absence of ligands, the dimer may recruit a corepressor, inhibiting PPARα-mediated transcription of target genes. The presence of an agonist, or an activator such as PGI2, triggers the recruitment of a coactivator complex which induces transcriptional activity of PPARα onto its target genes. This leads to an increase in fatty acid catabolism and adenosine triphosphate (ATP) production, also to decrease the levels of cytotoxic fatty acid peroxidation (POx) products, and, consequently, to promote cell viability and inhibit renal epithelium cell death. In addition, PPARα complex can attenuate NFκB-induced inflammatory factors (IL-6, INFγ, or TNFα) induced by ischemic/reperfusion injury (I/R) or drugs. Furthermore, PPARα complex can inhibit masengial matrix proliferation induced by TGFβ or reactive oxidative stress (ROS) which then resulted in albuminuria. After SUMOylation of PPARα, SUMOylated PPARα resulted in downregulation of its transcriptional activity by promoting its interaction with the corepressor NCoR, which will compromise cell viability and activate cell death processes. CoAct, coactivator; DM, diabetes mellitus; FAO, fatty acid oxidation; FFA, free fatty acid; IFNγ, interferon γ; IL-6, interleukine-6; I/R, ischemia/reperfusion; NCoR, nuclear corepressor; NF-κB, nuclear factor-κB; PGI2, prostacyclin; POx, peroxidation; PPARα, peroxisome proliferator-activated receptor-α; RXR, retinoid X receptor; TGFβ, tumor growth factor β; TNFα, tumor necrosis factor α.