Table 1.
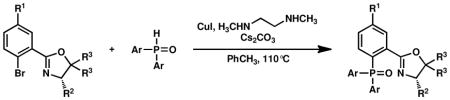
Copper-catalyzed coupling of aryl bromides with secondary phosphine oxides.a
 | ||||
|---|---|---|---|---|
| Entry | Aryl bromide | Secondary phosphine oxide | Product | Yield (%)b |
| 1 2c |
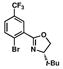 4 |
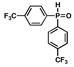 5 |
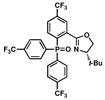 9 |
57 65 |
| 3 |
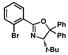 10 |
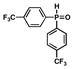 5 |
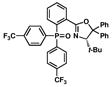 14 |
85 |
| 4 |
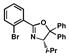 11 |
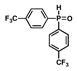 5 |
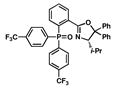 15 |
62 |
| 5 |
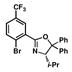 12 |
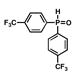 5 |
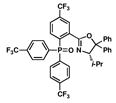 16 |
55 |
| 6 |
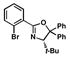 10 |
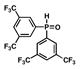 13 |
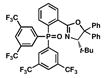 17 |
46 |
| 7 |
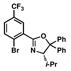 12 |
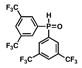 13 |
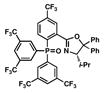 18 |
36 |
Reactions were performed with aryl bromide (1.0 equiv), secondary phosphine oxide (1.3 equiv), CuI (12.5 mol %), N,N′-dimethylethylenediamine (87.5% mol %), and Cs2CO3 (3.7 equiv) in PhCH3 (0.1 M) at 110 °C for 38–42 h.
Yield of isolated product.
Reaction was performed with 1.0 equiv of CuI and 3.0 equiv of N,N′-dimethylethylenediamine in PhCH3 (0.25 M) for 15 h.
