Abstract
Twelve dogs had transplantation of almost the entire small intestine in the orthotopic location; immunosuppression was with cyclosporine and prednisone. Half the dogs had survival of at least one month, and a third lived for at least four months. Two of the animals are still living after 550 and 555 days. Maintenance of nutrition, and absorption of D-xylose and fat were better than in control animals with an iatrogenic short gut syndrome, but distinctly worse than that of normal dogs.
Techniques of homotransplanting the small intestine alone (1) or as part of a multiple organ graft (2) were worked out in dogs almost a quarter of a century ago. However, Lillehei et al. (3) and others who followed (4–8) were not able to achieve chronic survival with the so-called conventional techniques of immunosuppression involving azathioprine and prednisone.
In the early autumn of 1981, our own first trials were undertaken with small bowel homotransplantation in dogs under cyclosporine and prednisone. Almost immediately it was obvious that long-term control of intestinal rejection was going to be feasible. About a month later at the Surgical Forum sessions of the American College of Surgeons, Craddock et al. (9) presented canine experiments that had been going on for more than a year. The report contained several examples of chronic survival, the longest for ten months.
Our own limited trials of small bowel transplantation in dogs were completed more than a year ago. The results will be described here with emphasis upon two animals who are 550 and 555 days postoperative, and upon absorption studies in the long-term survivors.
MATERIALS AND METHODS
Forty-two unrelated mongrel dogs of both sexes weighing 20–25 kg were used. Sixteen of the dogs were donors, and the others made up the recipient or control populations.
Experimental groups
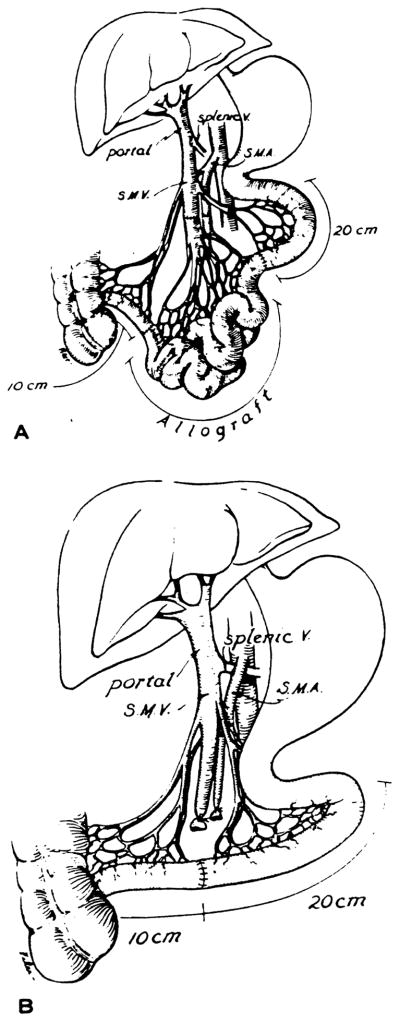
Group 1 (n = 12): The small intestine was resected leaving only 20 cm of the proximal jejunum and 10 cm of the distal ileum. The dogs immediately received orthotopic homografts of the entire jejunum and ileum (Fig. 1A) by a modification of Lillehei’s technique (3). The intestines were chilled by perfusion through the superior mesenteric artery with 1000 ml of cold lactated Ringer’s solution at 4 C containing 500 mg of Kanamycin. The intestinal lumen was flushed out with the same solution and the grafts were preserved by cold storage for 2–3 hr. The allografts were revascularized in the recipients by performing end-to-end anastomoses between the donor and recipient superior mesenteric arteries and veins (Fig. 1A). Intestinal continuity was restored by performing 2 layer, end-to-end, primary intestinal anastomoses. All dogs received oral immunosuppression with cyclosporine at a dose of 17 mg/kg/day and prednisone 20 mg/day, beginning on the first postoperative day. Penicillin and gentamycin were given parenterally for three days.
Figure 1.
Schematic depiction of the surgical procedures performed in groups 1 and 2 (A) and in group 3 (B).
Group 2 (n = 4): Four dogs underwent a similar surgical procedure and the same postoperative care but did not receive any immunosuppression (Fig. 1A).
Group 3 (n = 10): Massive intestinal resection was performed leaving the same amount of small bowel as in group 1. The intestinal continuity was restored between the jejunum and remaining 10 cm of ileum by performing end-to-end 2-layer anastomosis. These animals did not have transplantation (Fig. 1B).
Alimentation
All of the dogs received i.v. infusions during the first five postoperative days. However they were allowed to drink water ad libitum beginning on the second or third day and to eat solid food on the fifth postoperative day. Diet thereafter was with standard kennel rations.
Postoperative studies
Absorption of D-xylose and fat was determined in twenty dogs before they were assigned to any experimental group. The results were used as controls and were compared with the values at three and at nine months postoperatively in the animals of groups 1 and 3 that survived this long. In all three groups, body weights were determined at frequent intervals.
Liver function tests, including serum albumin, were obtained periodically. Renal function was assessed with serum creatinine or blood urea nitrogen measures. Serum electrolyte concentrations as well as calcium and phosphorus were measured.
RESULTS
Survival
Half the animals in Group 1 lived for a month or more and one-third survived for more than four months—the two longest survivors still being alive after 550 and 555 days (Table 1). In contrast all of the nonimmunosuppressed animals in group 2 rejected their grafts and died after 9–17 days. Six of the first seven dogs of group 3 died of starvation. Although three of the dogs in group 3 are still alive, they are extremely emaciated and are not expected to live much longer. The animals of group 3 had voracious appetites but had poor absorption.
Table 1.
Postoperative survival
| Group 1 (n = 12) |
Group 2 (n =4) |
Group 3 (n = 10) |
|||
|---|---|---|---|---|---|
| Survival (days) | Cause of death | Survival (days) | Cause of death | Survival (days) | Cause of death |
| 6 | Peritonitis from suture line leak | 9 | Rejection | 0 | Intraoperative |
| 27 | Peritonitis from partial graft gangrene | 9 | Rejection | 39 | Starvation |
| 3 | Pneumonia | 10 | Rejection | 42 | Starvation |
| 30 | Pneumonia | 17 | Rejection | 103 | Starvation |
| 15 | Septic thrombophlebitis | 150 | Starvation | ||
| 15 | Rejection | 292 | Starvation | ||
| 22 | Rejection and graft necrosis | 508 | Starvation | ||
| 43 | Rejection | 340 | Still alive | ||
| 118 | Rejection | 363 | Still alive | ||
| 125 | Rejection | 364 | Still alive | ||
| 550 | Still alive | ||||
| 555 | Still alive | ||||
Causes of death
These are listed in Table 1. In group 1, the lethal complications were those of immunosuppression and infections on one hand, or inadequate protection of the grafts and consequent rejection on the other. The dogs of group 2 rejected their grafts.
The appearance of lethal rejection, heralded by diarrhea and weight loss after four months in two animals of group 1, was disquieting. However, in the two dogs that are still living it is probable that rejection had also been present at an earlier time with reversal. During the second and third postoperative months both of these chronic survivors lost weight and had diarrhea. Subsequently their stools decreased in frequency and became normal in color and consistency as weight stabilized at about 85% of the original level (Fig. 2). The main cause of death in the animals of group 3 was starvation (Table 1) due to malabsorption.
Figure 2.
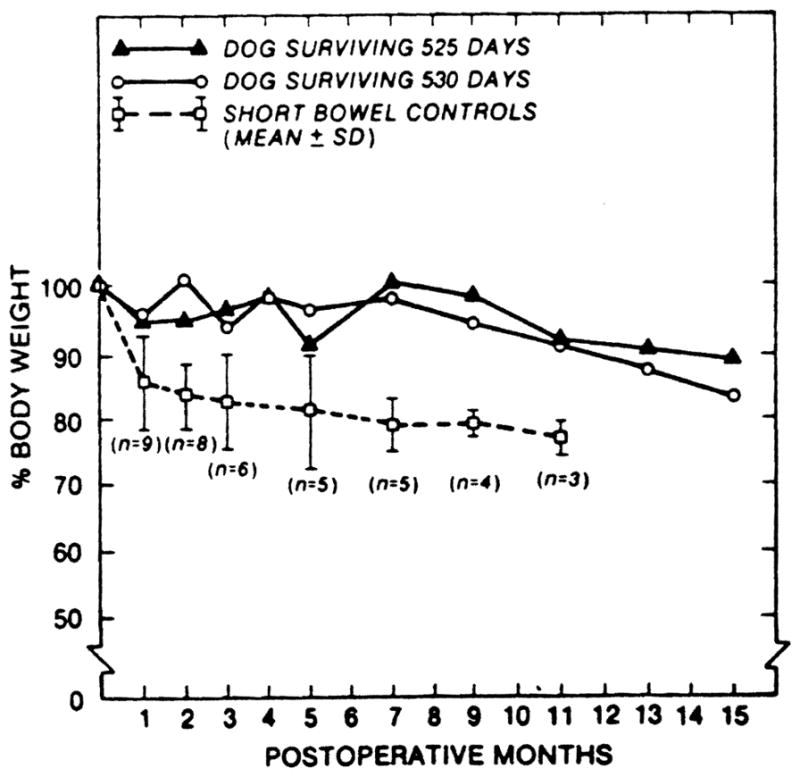
Body weight of the 2 long-term survivors of group 1 and short bowel controls (group 3).
In the animals of group 1 that died for reasons other than rejection, the pathologic findings in the graft were relatively minimal, with mild enlargement of the lymph nodes, and some thickening of the wall. In these animals the mucosa was intact.
In the animals that were thought to have died from rejection, the changes were quite heterogenous. Dogs that died early in the postoperative course had diffuse hemorrhagic necrosis of the graft, involving the mucosa most severely. Grossly, these early grafts were thin-walled with flattening and sloughing of the mucosa. Microscopically, changes ranged from coagulation necrosis of the mucosa to a decreased number of the epithelial cells and focal glandular hyperplasia. Mononuclear cell infiltration consisted of small and slightly immature lymphocytes and occasional plasma cells.
In dogs that died after the second postoperative month, the bowel tended to be thickened and the mucosa had a gross “cobblestone” pattern that was more noticeable toward the distal ileum. Microscopically there was focal atrophy of the villae with fibrosis of the lamina propria and a decrease in the number of glands. A mild lymphocyte infiltration was present around the myenteric plexuses and the serosa. The mucosa did not show an increased number of mononuclear cells in the lamina propria in most animals.
Laboratory studies
The weekly postoperative hematologic and biochemical studies in the various animals groups were pooled and averaged (Table 2). The dogs that rejected their grafts with (group 1) or without (group 2) immunosuppressive therapy tended to be anemic, and had elevations of the white blood cell counts, and extreme hypoalbuminemia. Serum lactic dehydrogenase and alkaline phosphatase concentrations were elevated in all of the transplant groups, with or without immunosuppression and whether or not rejection occurred; the dogs of group 3 with the iatrogenic short gut syndrome did not have these findings.
Table 2.
Laboratory data for various groups of dogs
| Normal (mean ± SD) | Group 1 |
Group 2 (Mean ± SD) | Group 3 (Mean ± SD) | ||
|---|---|---|---|---|---|
| No rejection (Mean ± SD) | With rejection (Mean ± SD) | ||||
| Hb(g/dL) | 15.2 (±2.3) | 14.4 (±1.2) | 10.9 (±0.8) | 11.4 (±1.1) | 13.1 (±1.9) |
| WBC(×103/ml) | 10.9 (±3.1) | 10.4 (±6.2) | 26.9 (±12.8) | 30.4 (±5.1) | 6.8 (±2.3) |
| Na (mEq/L) | 148.2 (±4.1) | 142.0 (±6.3) | 136.8 (±13.9) | 131.0 (±11.3) | 146.0 (±4.3) |
| Ca (mg/dl) | 10.7 (±0.6) | 9.5 (±0.7) | 8.4 (±1.0) | 8.9 (±0.4) | 9.3 (±0.4) |
| Albumin (g/dl) | 3.1 (±0.2) | 2.7 (±0.5) | 1.9 (±0.5) | 2.1 (±0.1) | 2.3 (±0.4) |
| A. Phosphatase (U/L) | 57.2 (±22.4) | 81.7 (±59.4) | 120.0 (±39.6) | 247.0 (±248.0) | 61.4 (±56.3) |
| LDH (U/L) | 92.8 (±30.3) | 209.0 (±47.5) | 242.0(±107.6) | 465.0 (±237.3) | 84.5 (±37.1) |
The dogs of group 1 with chronic survival after transplantation under immunosuppression maintained their weight (Fig. 2), serum albumin concentration (Table 2), D-xylose absorption (Figs. 3 and 4), and fat absorption (Fig. 5) better than the animals of group 3 with the iatrogenic short gut syndrome. However, even in the long-surviving graft recipients, all these measures were subnormal compared with those in the normal canine control population, and they were only slightly better than in the animals of group 3 with the short gut syndrome.
Figure 3.
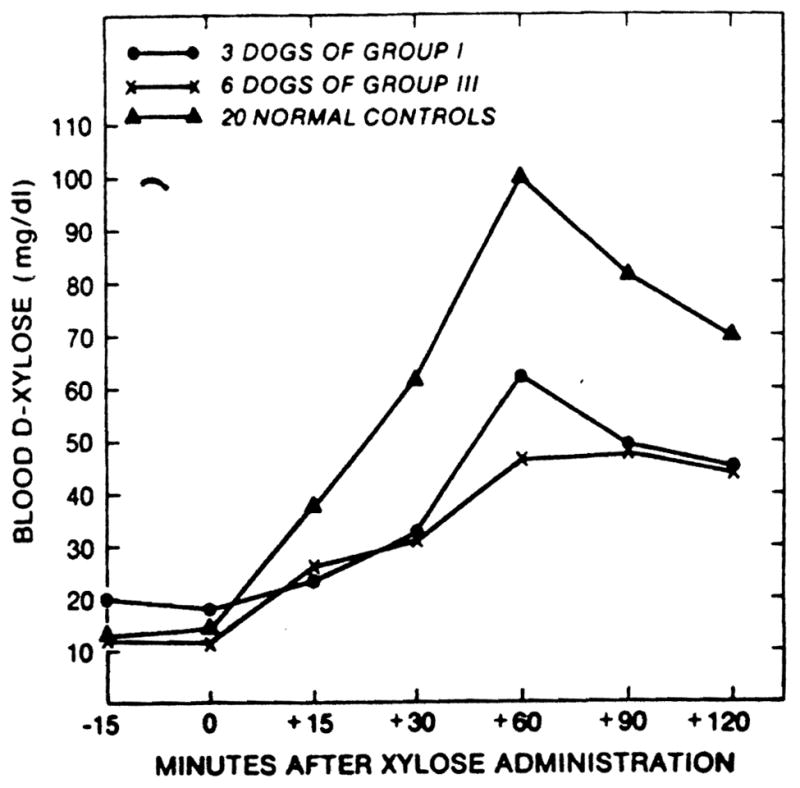
D-xylose absorption test at 3 months postoperatively.
Figure 4.
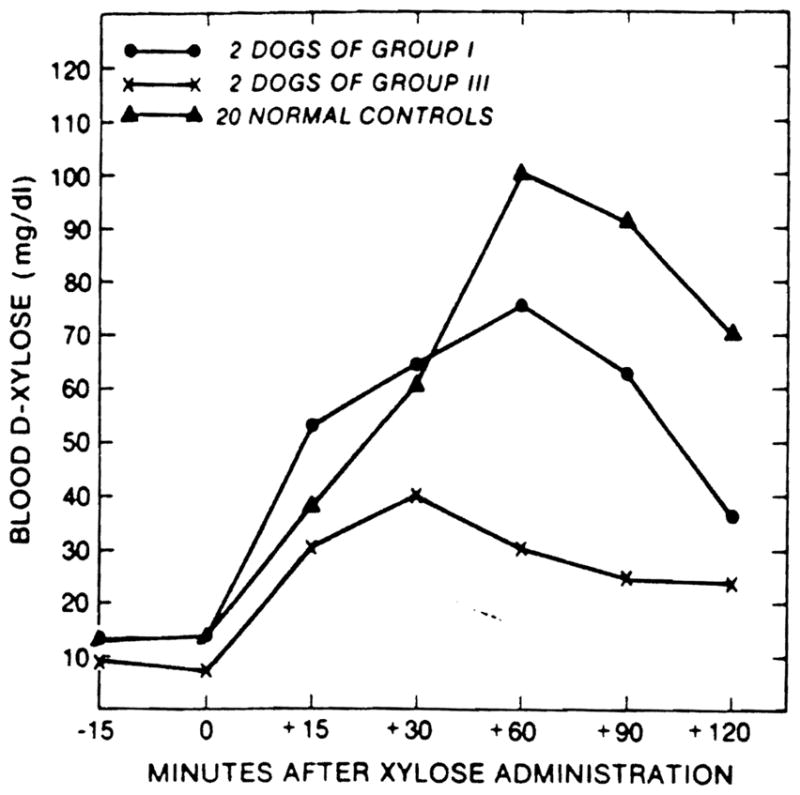
D-xylose absorption test at 9 months postoperatively.
Figure 5.
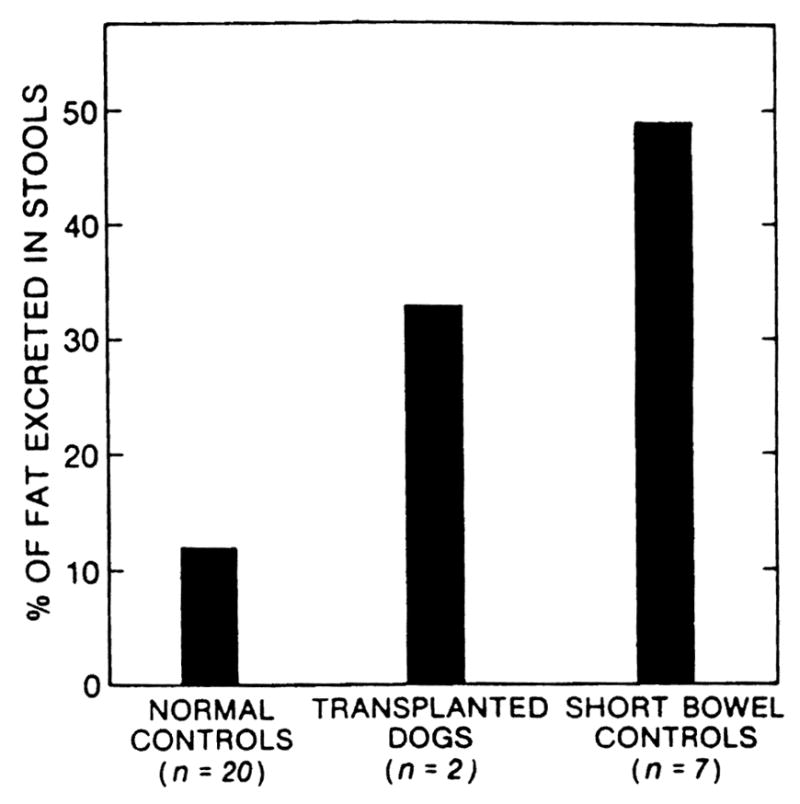
Fecal fat excretion at 3 months postoperatively.
DISCUSSION
These studies, as well as those of Craddock et al (9), have shown for the first time that small bowel transplantation in dogs is a feasible undertaking. In spite of the fact that the intestinal grafts had to transmit food and intestinal contents from the day of operation onward, extended survival was obtained in half the animals and survival beyond three months was achieved in one-third.
Despite this encouraging notation, the intestinal homografts had distinctly abnormal function, even in the two animals with the longest survivals. The chronically surviving animals appeared to be healthy, but their serum albumin concentrations were intermediate between those of normal dogs and the animals with the iatrogenic short gut syndrome. The absorption of D-xylose and fat followed the same imperfect pattern, and this was reflected in the fact that the weight of the two animals that are still alive after 18 months fell to a lower but eventually stable level postoperatively.
Findings such as those reported here encourage the hope that clinical transplantation of the small bowel is within grasp, but unanswered questions remain. For example, it is not known how well the intestinal homografts absorb the oral cyclosporine upon which continued prevention of rejection depends. To the extent that absorption of the drug is defective, late difficulties with rejection may be anticipated, and this could have been the explanation for the abrupt rejection of at least two grafts after many months of seemingly satisfactory intestinal function. In addition, it may become important to know whether anastomosis of the intestinal venous return into the portal circulation is important. In our experiments, the intestinal grafts were in the orthotopic location, but in patients needing intestinal transplantation it is likely that for technical and anatomical reasons the venous outflow of the grafts must be directed into the systemic circulation by anastomosis to the inferior vena cava, thus bypassing the liver.
Footnotes
This work was supported by research grants from the Veterans Administration; by project grant AM-29961 from the National Institutes of Health; and by grant RR-00084 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
LITERATURE CITED
- 1.Lillehei RC, Groot B, Miller FA. Homografts of the small bowel. Surg Forum. 1959;10:197. [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillehei RC, Idezuki Y, Feemster JA, et al. Transplantation of stomach, intestine, and pancreas: experimental and clinical observations. Surgery. 1967;62(4):721. [PubMed] [Google Scholar]
- 4.Holmes JT, Yeh SDJ, Winawer SJ, Kawano N, Fortner JG. Absorption studies in canine jejunal allografts. Ann Surg. 1971;174:101. doi: 10.1097/00000658-197107010-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alican F, Hardy JD, Cayirli M, et al. Intestinal transplantation: laboratory experience and report of a clinical case. Am J Surg. 1971;121:150. doi: 10.1016/0002-9610(71)90092-4. [DOI] [PubMed] [Google Scholar]
- 6.Hardy MA, Quint J, State D. Effect of antilymphocyte serum and other immunosuppressive agents on canine jejunal allografts. Ann Surg. 1970;171:51. doi: 10.1097/00000658-197001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preston FW, Macalalad F, Wachowski TJ, Randolph DA, Apostol JV. Survival of homografts of the intestine with and without immunosuppression. Surgery. 1966;60 (6):1203. [PubMed] [Google Scholar]
- 8.Taylor RMR, Watson JW, Walker FC, Watson AJ. Prolongation of survival of jejunal homografts in dogs treated with azathioprine (Imuran) Br J Surg. 1966;53(2):134. doi: 10.1002/bjs.1800530212. [DOI] [PubMed] [Google Scholar]
- 9.Craddock GN, Reznick RK, Gilas T, Langer B, Wolman WG, Cullen JB. Structure and function of small bowel allografts treated with cyclosporin A. Surg Forum. 1981;32:350. [PubMed] [Google Scholar]