Testing for cytotoxic antibodies in recipient serum, against donor lymphocytes, is perhaps the most important test done prior to allotransplantation. With the possible exception of liver transplants, a positive T cell crossmatch at 37 C is generally considered to be an absolute contraindication for organ transplantation (1, 2). The status of other types of positive crossmatches remains to be clarified (3,4). Allotransplantation done in the presence of a positive, warm T cell crossmatch does not always result in immediate graft loss, although hyperacute rejection or accelerated rejection is frequent.
Both hyperacute rejection and graft survival have been described in cardiac transplants done in the presence of a positive crossmatch (5, 6). We observed an intermediate outcome with early graft injury without immediate loss of the organ in a patient who received a cardiac transplant from a crossmatch-positive donor. The relevant clinical data are presented below:
MB, a 48-year-old white woman, presented with severe congestive heart failure requiring vasopressors immediately following cardiac catheterization. Coronary arteries were normal and a diagnosis of cardiomyopathy with biventricular dysfunction was made. Cardiac transplantation was done on the 5th hospital day (recipient HLA type A2,A24 (9),B7,B27,DR2; donor HLA type A3,A26(10),B15,B22,DR2,DR7).
The recipient suffered a cardiac arrest within 2 hr of transplantation, but she was successfully resuscitated. She was treated with cyclosporin A and prednisolone. Cyclosporin A was given orally at a dose of 17.5 mg/kg/day for 19 days when a rise in bilirubin was observed and the dose reduced to 6.5 mg/kg/day. She received 1 g of methylprednisolone at surgery and 1 g of cortisol i.v., every 4 hr for 24 hr. On the 2nd postoperative day, she received 200 mg of prednisone orally. The prednisone dose was reduced by 20 mg/day until 30 mg/day was reached.
The donor heart was obtained from a distant city and the crossmatch between donor lymph node cells and the recipient serum was completed after transplantation. The crossmatch was carried out by the two-color fluorescence method at 4 and 37 C (7; Rabin et al., unpublished data). The crossmatch was positive at both temperatures against both T and B lymphocytes at a serum dilution of 1:4. Following a single platelet absorption of the patient’s serum, the crossmatch was negative.
The patient’s pretransplant serum was tested for the presence of lymphocytotoxic antibodies using a 26-member random panel by the trypan blue dye exclusion method. The patient’s serum was cytotoxic to T and B lymphocytes from 50% of the panel members.
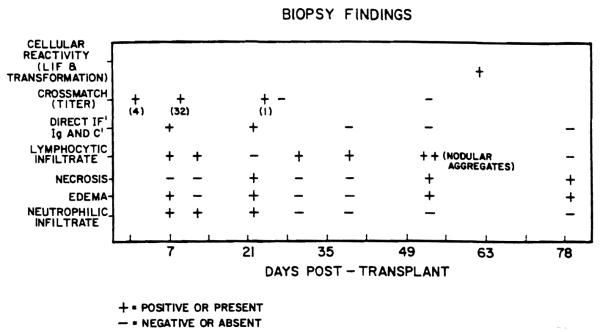
Three transvenous endomyocardial biopsies were done each month during the 2-month post-transplant period. The results of biopsy findings are outlined in Figure 1. The biopsy taken 1 week after transplantation showed focal lymphocytic and neutrophilic infiltration with edema. The changes were felt to be consistent with mild acute rejection. The biopsy taken 22 days after transplantation exhibited edema with mild neutrophilic infiltration and focal myocytolysis without any appreciable lymphocytic infiltration. The biopsy taken 53 days after transplantation showed moderate, acute cellular rejection with nodular aggregates of lymphocytes, focal myocytolysis, and endocardial fibrosis.
Figure 1.
Biopsy findings: The type test done is indicated on the vertical axis and the post-transplant days on which the tests were conducted are indicated on the horizontal axis. Necrosis myocardial fibers, edema, neutrophilic and lymphocytic infiltrate assessed from biopsy specimens processed for light microscopy. Direct immunofluorescence was done on separate specimens processed as frozen sections. Crossmatch titers indicate the serum dilution at which more than 80% of the donor lymphocytes were killed. Cellular reactivity and transformation pertain to the in vitro tests with saline extract of donor heart tissue.
Four of the biopsies were examined by direct immunofluorescence for IgG, IgM, IgA, C3, C4, Clq, properdin, and fibrinogen. Vascular deposition of IgG and C3 were present in the biopsy taken 1 week after transplantation (Fig. 2). IgG, IgM, C3, C4, and fibrinogen deposits were seen in the vessel walls in the biopsy taken 22 days postoperatively. The succeeding biopsies were negative by direct immunofluorescence (Fig. 1).
Figure 2.
The endomyocardial biopsy was stained with fluorescein-conjugated antihuman C3 antibody. The deposition of C3 in small vessels is evident. IgG, IgM, C4, and fibrinogen deposition were seen in similar distribution.
Postoperative serum specimens were examined for the presence of antibodies cytotoxic to donor cells by the same technique used for the original crossmatch. The serum taken 9 days post-transplantation gave a positive, warm T cell crossmatch with a titer of 32, a specimen taken 24 days post-transplantation was positive only when tested undiluted. The serum specimens taken 27 and 53 days post-transplantation were negative for donor lymphocyte-specific cytotoxic antibodies (Fig. 1).
The patient’s lymphocytes were tested (62 days post-transplantation) for their reactivity to donor heart tissue. The stimulation index, on incubating the patient’s lymphocytes with saline extract of donor heart tissue, was 5.7 whereas the index in response to third-party liver extract was 2.4. The patient’s lymphocytes were also tested for their ability to produce leukocyte migration inhibition factor in an indirect assay using saline extract of donor heart and third-party liver extract. The migration inhibition index with the donor heart extract was 0.64. The patient’s lymphocytes, on exposure to third-party liver extract, and lymphocytes from a normal donor on exposure to donor heart and third-party liver extract, failed to produce leukocyte migration inhibition factor.
The patient’s EKGs demonstrated reduction in voltage in the early post-transplant period. However, the output stabilized by the 30th post-transplant day. The patient was discharged in a stable, though not a satisfactory, condition 8 weeks post-transplantation. She expired 2 weeks later with a 7-cm mediastinal abscess and bilateral pneumonia. Histologically, the ventricular myocardium showed edema and mild focal individual cell necrosis. The vascular endothelial cells were prominent, but no evidence of accelerated atherosclerosis was present.
The patient’s course, especially the study of endomyocardial biopsies (Fig. 1), points to a transient vascular injury initiated by the deposition of alloantibodies in addition to acute cellular rejection episodes. The outcome, however, was not hyperacute rejection as is the usual case with preformed antibodies. Although the treatment may have altered the appearance of the lesions, the histological findings during the period when direct immunofluorescence was positive were not entirely consistent with cell-mediated acute rejection, especially around postoperative days 22 to 24. The early picture can thus be described as an alloantibody-mediated vasculitis with mild acute rejection, leading to transient graft injury with recovery. We have no explanation for the nonprogressive nature of the vasculitis or the disappearance of the preformed antidonor antibodies in the patient’s serum. Hypogammaglobulinemia did not occur during the course of observation. Although we were unable to serially test the cellular response to donor tissue, the single test (mitogenic response and leukocyte migration inhibition factor production) was positive at a time when biopsy findings indicated acute cellular rejection.
Despite general agreement that allotransplants should not be done when a warm T cell crossmatch is positive, there may not be enough time to wait for the results of the crossmatch because of limited tolerance of the organ to ischemia. The indication of the presence of antiallotypic antibodies in the patient’s serum can be obtained by testing the serum against a panel of lymphocytes. The use of a panel of lymphocytes frozen and stored in Terasaki tissue typing trays was found to expedite such screening (8). It is being suggested that quick screening should be carried out for all prospective recipients to estimate the probability of a positive crossmatch against a random donor. By using lymphocytes frozen in Terasaki trays, a quick screening could be carried out for all patients within a matter of about 5 hr. A patient reacting with 50% of the panel members has roughly a 50% chance of having a positive crossmatch with a random donor. It may, at times, not be possible to wait for the crossmatch results before transplantation; under such circumstances, knowing the probability of a positive crossmatch against a random donor may facilitate the decision for undertaking or not undertaking a transplant. We are in agreement with the recommendation of Weil et al. (5) that cardiac transplantation should not be done in the presence of a positive, warm T cell crossmatch unless the patient is not likely to survive long enough to wait for another organ.
In summary, we have described a patient who had cytotoxic antibodies to 50% of the members of a random panel and had a positive, warm T cell cross match with the donor lymphocytes. The cardiac transplant suffered transient vasculitis, but did not undergo hyperacute rejection. Evidence for humoral rejection. i.e., positive direct immunofluorescence and positive crossmatches, waned despite initial positivity. Humoral and cellular rejection were present simultaneously in the initial period, but were discordant after the initial 2 weeks. It is suggested that quick screening for lymphocytotoxic antibodies be carried out using lymphocytes frozen in Terasaki typing trays to determine the likelihood of a positive crossmatch. Whenever possible, transplantation in the face of a positive crossmatch should be avoided.
Acknowledgments
We are grateful to Dr. N. R. Dunn and her staff for their kind cooperation.
LITERATURE CITED
- 1.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from crossmatch-positive donor. Transplant Proc. 1981;13:286. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel R, Terasaki PI. Significance of the positive crossmatch in kidney transplantation. N Engl J Med. 1969;280:735. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 3.Guttmann RD. Renal transplantation. N Engl J Med. 1979;301:975. doi: 10.1056/NEJM197911013011805. [DOI] [PubMed] [Google Scholar]
- 4.Jeannet M, Benzonana G, Arni I. Donor-specific B and T lymphocyte antibodies and kidney graft survival. Transplantation. 1981;31:160. doi: 10.1097/00007890-198103000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Weil R, III, Clarke DR, Iwaki Y, et al. Hyperacute rejection of a transplanted human heart. Transplantation. 1981;32:71. [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner WA, Reitz BA, Oyer PE, Stinson EB, Shumway NE. Cardiac homotransplantation. Curr Probl Surg. 1979;16:1. doi: 10.1016/s0011-3840(79)80010-6. [DOI] [PubMed] [Google Scholar]
- 7.van Rood JJ, van Leeuwen A, Plovern JS. Simultaneous detection of two cell populations by two-colour fluorescence and application to the recognition of B-cell determinants. Nature. 1976;262:795. doi: 10.1038/262795a0. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein AS, Hubbard MC, Barry JM. A simple method for freezing lymphocytes in microtest plates. Am J Clin Pathol. 1981;75:221. doi: 10.1093/ajcp/75.2.221. [DOI] [PubMed] [Google Scholar]