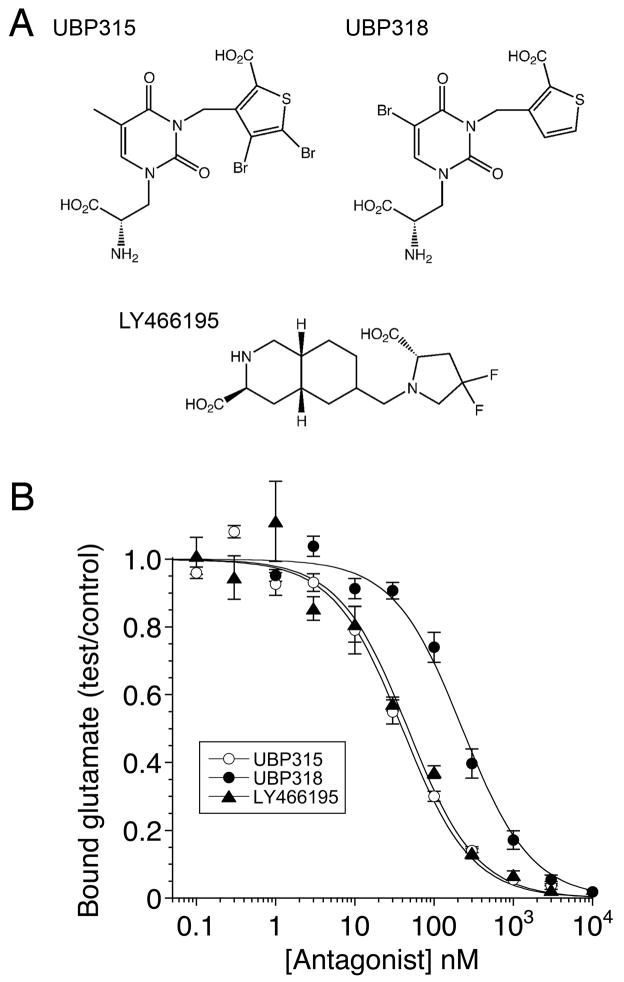
Figure 1.
Structures and binding assays for GluK1 competitive antagonists. (A) UBP315 and UBP318 are willardiine derivatives with bromine substitutions on the thiophene and uracil rings respectively; LY466195 is a decahydroisoquinoline with fluorine substitutions on the pyrrolidine ring. (B) Radioligand displacement curves for the purified GluK1 ligand binding domain showing competition between 3[H]-L-glutamate and three antagonists, fit with single binding site isotherms of 33 ± 4 nM, 186 ± 23 nM and 38 ± 7 nM for UBP315, UBP318 and LY466195, respectively; values are mean ± SEM of triplicate measurements.