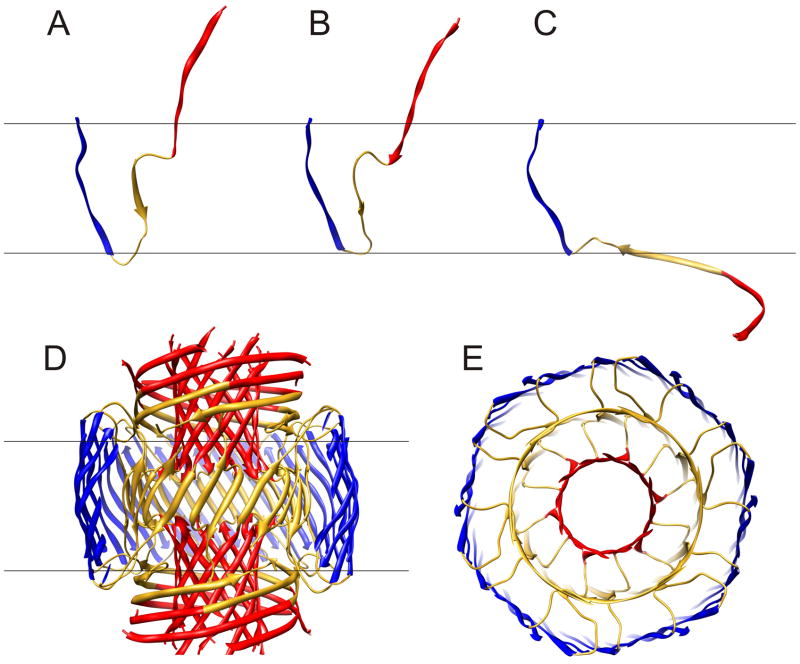
Figure 4.
Ribbon representation of the final stage model in which blue S3 segments form a 36-stranded antiparallel β-barrel (same as model in Fig. 3E). (A–C) Conformations of the three types of subunits, Sub1, Sub2, and Sub3, that comprise the model; 12 copies of each are in the complete model. (D) Side view of the complete model. Some of the S3 strands nearest the viewer have been clipped off to reveal the structure of the core region. Red S1 segments form two 12-stranded parallel β-barrels, central hydrophobic residues (17–21) of gold S2 segments form a central 24-stranded anitparallel β-barrel. (E) Half of the transmembrane region viewed from the aqueous phase along the central axis of the pore.