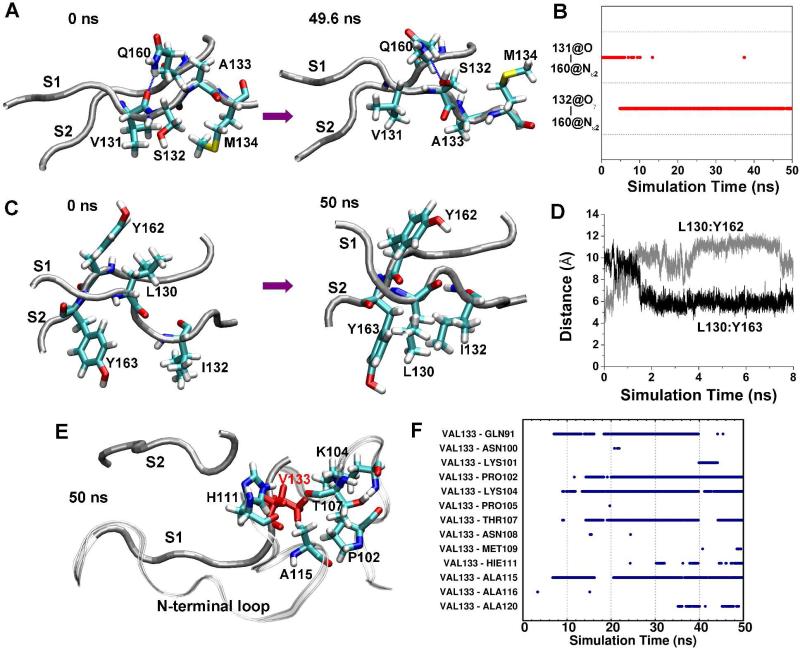
Figure 6.
Reconfiguration of side chain interactions of the native sheet due to mutations. A. Conformations of strands S1 and S2 at the indicated times in run 1 of PrP mutant G131V. The backbones of the strands are shown as tubes and relevant residues are represented as sticks and labeled, as is the case in panels C and E. B. Time course of hydrogen bonds between S131:O and Q160:Nε2 and between S132:Oγ and Q160:Nε2 in run 1 of PrP mutant G131V. C. Conformations of strands S1 and S2 at indicated times in run 2 of PrP mutant S132I. D. The distance between the centers of mass of the L130 side chain and the Y162 side chain in run 2 of PrP mutant S132I. E. The structure of the S1, S2 strands, and N-terminal region at 50 ns in run 2 of PrP mutant A133V. V133 is in red. The backbone of the N-terminal region is shown as a ribbon. F. Time course of hydrophobic side chain-side chain contacts between V133 and residues of the N-terminal region in run 2 of PrP mutant A133V.