Abstract
Background
Immunosuppressed patients are at increased risk for herpes zoster (HZ), but incidence in solid organ transplant (SOT) recipients has varied in multiple studies. To assess incidence of HZ, we examined patients who underwent SOT and received follow-up care within the large multicenter Us Department of Veteran’s Affairs Healthcare system.
Methods
Incident cases of HZ were determined using ICD-9 coding from administrative databases. A multivariable Cox proportional hazards model, adjusted for a priori risk factors, was used to assess demographic factors associated with development of HZ.
Results
Among the 1077 eligible SOT recipients, the cohort-specific incidence rate of HZ was 22.2 per 1000 patient-years (95% confidence interval [CI], 18.1–27.4). African Americans (37.6 per 1000 [95% CI, 25.0–56.6]) and heart transplants recipients (40.0 per 1000 [95% CI, 23.2–68.9]) had the highest incidence of HZ. Patients transplanted between 2005 and 2007 had the lowest incidence (15.3 per 1000 [95% CI, 8.2–28.3]). In a multivariable model, African Americans (hazard ratio [HR] 1.88; 95% CI: 1.12, 3.17) and older transplant recipients (HR 1.13; 95% CI: 1.01, 1.27 [per 5-year increment]) had increased relative hazards of HZ.
Conclusions
These data demonstrate that HZ is a common infectious complication following SOT. Future studies focused on HZ prevention are needed in this high-risk population.
Keywords: herpes zoster, infection, liver transplantation, kidney transplantation, African American
Background
Herpes zoster (HZ) is a frequent complication of varicella zoster virus infection, and occurs with an estimated annual incidence of 1.5 to 3.0 cases per 1000 persons-years (1). Up to 20% of individuals develop HZ during their lifetime (2), and rates are increased in the elderly and in those with impaired cell-mediated immunity or underlying malignancy (1, 3, 4). These susceptible populations may also be at increased risk for post-herpetic neuralgia and other complications of HZ (5).
The number of solid organ transplants (SOTs) in the United States has increased over the past few decades (6, 7) and improvements in immunosuppression and post-transplant management have increased allograft survival (8). Because of the need for lifetime immunosuppression, SOT recipients are at increased risk for developing HZ. However, because of variations in immunosuppressive regimens, organ transplanted, antiviral prophylaxis, and other center effects, results in single-center prevalence estimates of HZ in SOT patients vary anywhere from 1.5% –16.2% (9–16). A large multi-center study evaluating overall incidence could help to confirm findings seen at individual centers.
The US Department of Veteran’s Affairs (VA) healthcare system patient population is of considerable interest, because of its large size and national representation. The VA healthcare system is the largest single-payer healthcare system in the United States (17) and vulnerable populations, such as underrepresented minorities and people with low incomes are present in substantial numbers in this population (18). The VA began offering SOTs to veterans in 1961, and currently provides access to kidney, liver, heart, pancreas, and lung transplants. We conducted a multicenter retrospective population-based cohort study using the VA’s large administrative database, in order to assess the incidence of HZ in SOT recipients.
Materials and methods
Patient population
The VA has maintained electronic records on all patients treated in VA facilities for >30 years, and their database currently contains longitudinal records on all persons seeking care in >150 federal hospitals and nearly 900 outpatient clinics (17). We accessed these electronic records to identify a cohort of veterans who had undergone their first SOT at a VA-affiliated hospital between January 1995 and December 2007. Eligible patients were identified using specific transplant surgery International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) procedure codes associated with specific organ transplants (ICD-9 codes 33.5 [lung], 33.6 [combined heart lung], 37.5 [heart], 50.5 [liver], 52.8 [pancreas], and 55.6 [kidney]). Persons who survived <14 days after transplant, non-veterans, and those who underwent transplantation at non-VA facilities but who received follow-up care within the VA were excluded from all analyses.
Information on demographic factors, hospitalizations, and outpatient care were ascertained for the cohort from VA electronic medical records contained in an administrative database. Demographic information included age at transplantation, sex, race and ethnicity, and year of transplantation. Since VA transplant centers are located in Oregon, Pennsylvania, Iowa, Texas, and 2 other non-VA hospitals contracted by the VA to perform liver transplants in Virginia and Tennessee, we also assessed geographic region of VA hospital where the transplant took place (East, Midwest, South, and West). To assess other co-morbidities, we calculated an adapted Charlson index for ICD-9 coding (Charlson-Deyo) (19). This index summarizes the presence or absence of 17 medical conditions, and a score of 0 means that none of these co-morbid conditions is present; a higher score indicates greater burden of comorbidity (20). For these analyses, this index was classified as having none, 1, 2, or ≥ 3 comorbidities (19, 20). We further identified organ rejection/failure (996.8 Complications of transplantation – transplant failure or organ rejection) and cytomegalovirus (CMV) disease (078.5 Cytomegaloviral disease), as both are common transplant complications that might alter patient outcomes and were available in this administrative dataset.
Available records were also used to determine VA service connection, defined as a disability or illness that is considered a result of or aggravated by military service (21). To evaluate differences in this variable, we specified whether patients had service-connected disability (>=10% vs. < 10%). Additionally, war-time veterans can receive a pension if they are permanently and totally disabled from non-service connected disabilities and have very low income. We classified these patients as having received a pension for non-service connected disability. All others we considered to be non-service connected and without VA pension. Since patients are known to seek care outside of the VA system (22) and service connection strongly predicts the use of VA healthcare services (23), service connection can serve to adjust for VA healthcare use. In addition, service connection can be used as a proxy for socioeconomic status (24).
Outcome
An incident case of HZ was defined in patients newly linked with the ICD-9 code 053 (Herpes zoster) during either an inpatient or outpatient visit in the post-transplant period (25). If a patient developed recurrent HZ, they were only considered to have developed HZ with the primary episode in all primary analyses. Recurrent episodes of zoster were identified in patients with prior HZ who had a newly linked HZ ICD-9 code (except those associated with post-herpetic neuralgia) 180 or more days after primary diagnosis (26). We also indentified patients who developed post-herpetic neuralgia defined as the appearance of the following ICD-9 codes: 053.12 (post-herpetic trigeminal neuralgia), 053.13 (post-herpetic polyneuralgia) or 729.2 (neuralgia, neuritis, and radiculitis, unspecified) (27). These methods have been validated using the VA’s large administrative dataset in prior epidemiologic studies (28–32), and have been shown to have a positive predictive value of 90% for HZ (32). Inpatient codes were available prior to January 1995, but outpatient diagnostic codes only became available beginning on October 1, 1996. In order to account for these missing data, only patients with both outpatient and inpatient data were included in these analyses.
Statistics
The incidence rates of HZ were estimated by dividing the number of incident HZ cases developed in cohort subjects by the number of years of person-time at-risk (post transplant) contributed by the overall cohort; 95% confidence intervals (CI) were estimated based on a Poisson distribution. Rates were then stratified by age at transplant, race, type of organ transplanted, and calendar year transplanted. Incidence rates were also calculated at each year post transplant by excluding patients who had developed their first episode of HZ in the prior year and including events only during the assessed post-transplant year. Cumulative incidence curves were also used to estimate incidence of HZ during follow-up, censoring at last VA contact, and considering re-transplantation and death as competing risks. Cumulative incidence curves were compared between subgroups of race, organ transplanted, and calendar year of transplant using the log-rank test.
To estimate demographic risk factors for the development of post-transplant HZ, univariate and multivariable Cox proportional hazards models were used. For analyses, available a priori variables included age (per 5-year interval), race (black, white, other), type of transplant (kidney, liver, heart), transplant calendar year (modeled as a group linear term [Oct 1996–1999, 2000–2004, 2005–2007], and comorbidity index (0 to ≥3), organ rejection/failure, any CMV disease, and VA service-connection status (service connected and non-service connected). Organ rejection and CMV disease were added as time-dependent variables. Patients were censored at death, re-transplantation, or when lost to follow-up. All proportional hazards assumptions were evaluated by examining Schoenfeld residuals (33) and log-log plots of overall survival (34). All statistical analyses were completed using STATA version 10 (College Station, Texas USA). The study was approved by the University of Washington and VA Puget Sound Institutional Review Boards.
Results
We identified 1781 subjects that underwent SOT within the VA healthcare system from 1995–2007 using the VA administrative database. After exclusion of non-veterans (n=409), patients with missing outpatient data (n=205) and subjects who survived less than 14 days (n=90), a total of 1077 were eligible for analysis (Table 1). The median age at transplant for the cohort was 53.9 years (interquartile range [IQR] 48.2, 59.2). The cohort was made up primarily of men (n=1053 [98%]). The cohort was mostly white 741/1107 (69%) and African American 168/1254 (16%); there was a small number of other races (168/1254 [16%]). The majority of patients underwent a kidney (500 [46%]) or liver transplant (461 [43%]), while there were smaller numbers of those receiving heart (80 [7.4%]) and other organ transplants (36 [3.3%]). Most transplants occurred in the Southern and Eastern United States, likely reflecting the geographic distribution of transplant centers within the VA system.
Table 1.
Demographics of cohort of subjects transplanted at Veteran’s Health Affairs Hospitals from 1996–2007 (n=1077)
| Variables | Cohort n (%)1 |
|---|---|
| Time at risk | 1372 ± 1038 |
| Age (median [IQR]) | 53.9 [48.2, 59.2] |
| Sex (Male) | 1053 (97.8) |
| Race/ethnicity | |
| White | 741 (68.8) |
| African American | 168 (15.6) |
| Hispanic | 63 (5.9) |
| Asian | 9 (0.8) |
| Native American | 12 (1.1) |
| Other/Unknown | 84 (7.8) |
| Transplant type | |
| Kidney | 500 (46.4) |
| Liver | 461 (42.8) |
| Heart | 80 (7.4) |
| Others | 36 (3.3) |
| Year of transplant | |
| Oct 1996–1999 | 261 (24.2) |
| 2000–2004 | 439 (40.8) |
| 2005–2007 | 377 (35.0) |
| Comorbidity index2 | |
| 0 | 136 (12.6) |
| 1 | 155 (14.4) |
| 2 | 270 (25.1) |
| ≥3 | 516 (47.9) |
| VA status | |
| Service connected ≥10% | 377 (35.0) |
| Service connected <10% | 23 (2.1) |
| No service connection | 548 (50.9) |
| Pension | 129 (12.0) |
| Geographic region | |
| West | 254 (23.6) |
| Midwest | 114 (10.6) |
| South | 373 (34.6) |
| East | 336 (31.2) |
| Organ rejection3 | |
| Yes | 528 (49.0) |
| No | 549 (51.0) |
| CMV3, 4 | |
| Yes | 107 (9.9) |
| No | 970 (90.1) |
Because of rounding, percentages may not equal 100.
Adapted Charlson Index.
Before development of herpes zoster.
Any CMV event.
VA, US Department of Veteran’s Affairs healthcare system; IQR, Interquartile range; CMV, cytomegalovirus.
In this cohort, HZ developed in 90 (8.4%) of the 1077 subjects at a median of 2.6 years (IQR 0.87, 4.50) after transplantation. The incidence rate of HZ in those transplanted after October 1996 was 22.2 per 1000 patient-years (95% CI: 18.1, 27.4) over the post-transplant follow-up period (Table 2). In those patients developing HZ, 4/90 (4%) developed at least 1 recurrent episode 180 or more days after primary diagnosis. Additionally, 23/90 (26%) developed post-herpetic neuralgia.
Table 2.
Incidence rates of herpes zoster in cohort of solid organ transplant recipient Veterans from October 1, 1996–2007 (n=1077)1
| Variable | n | Person- years at risk |
Cases | IncidencerRate2 (95% CI) |
|---|---|---|---|---|
| Overall | 1077 | 4044 | 90 | 22.2 (18.1, 27.4) |
| Age | ||||
| <45 | 161 | 784 | 16 | 20.4 (12.5, 33.3) |
| 45 – 55 | 446 | 1815 | 36 | 19.8 (14.3, 27.5) |
| 55+ | 470 | 1445 | 38 | 26.3 (19.1, 36.1) |
| Race | ||||
| White | 741 | 2831 | 55 | 19.4 (14.9, 25.3) |
| African American | 168 | 612 | 23 | 37.6 (25.0, 56.6) |
| Other | 168 | 601 | 12 | 20.0 (11.3, 35.1) |
| Type of transplant | ||||
| Kidney | 500 | 1847 | 45 | 24.4 (18.2, 32.6) |
| Liver | 461 | 1751 | 32 | 18.3 (12.9, 25.8) |
| Heart | 80 | 325 | 13 | 40.0 (23.2, 68.9) |
| Year of transplant | ||||
| 1996–19993 | 261 | 1553 | 45 | 29.0 (21.6, 38.8) |
| 2000–2004 | 439 | 1835 | 35 | 19.1 (13.7, 26.6) |
| 2005–2007 | 377 | 657 | 10 | 15.2 (8.2, 28.3) |
| Year post transplant | ||||
| Year 1 | 1077 | 996 | 28 | 28.1 (19.4, 40.7) |
| Year 2 | 913 | 812 | 14 | 17.2 (10.2, 29.1) |
| Year 3 | 710 | 615 | 14 | 22.8 (13.5, 38.4) |
| Year 4 | 530 | 464 | 15 | 32.3 (19.5, 53.6) |
Excludes patients with survival ≤14 days and those with incomplete outpatient data.
Per 1,000 patient-years.
Patients only after October 1996.
CI, confidence interval.
Incidence rates were highest in African Americans and heart transplant recipients, while Whites and liver transplant recipients had the lowest incidence rates of HZ (Table 2). Too few women were included in this cohort to calculate incidence rates based on gender, and the limited number of Asian/Pacific Islanders and Native Americans in our population prevented evaluation of incidence in these populations. Also, owing to the small number of other organ transplant types (n=44), no comment could be made on the incidence in those with other transplanted organs. Of those patients excluded in incidence assessments for lack of outpatient data, 20/205 (10%) of patients were noted to have developed HZ at some point during their follow-up.
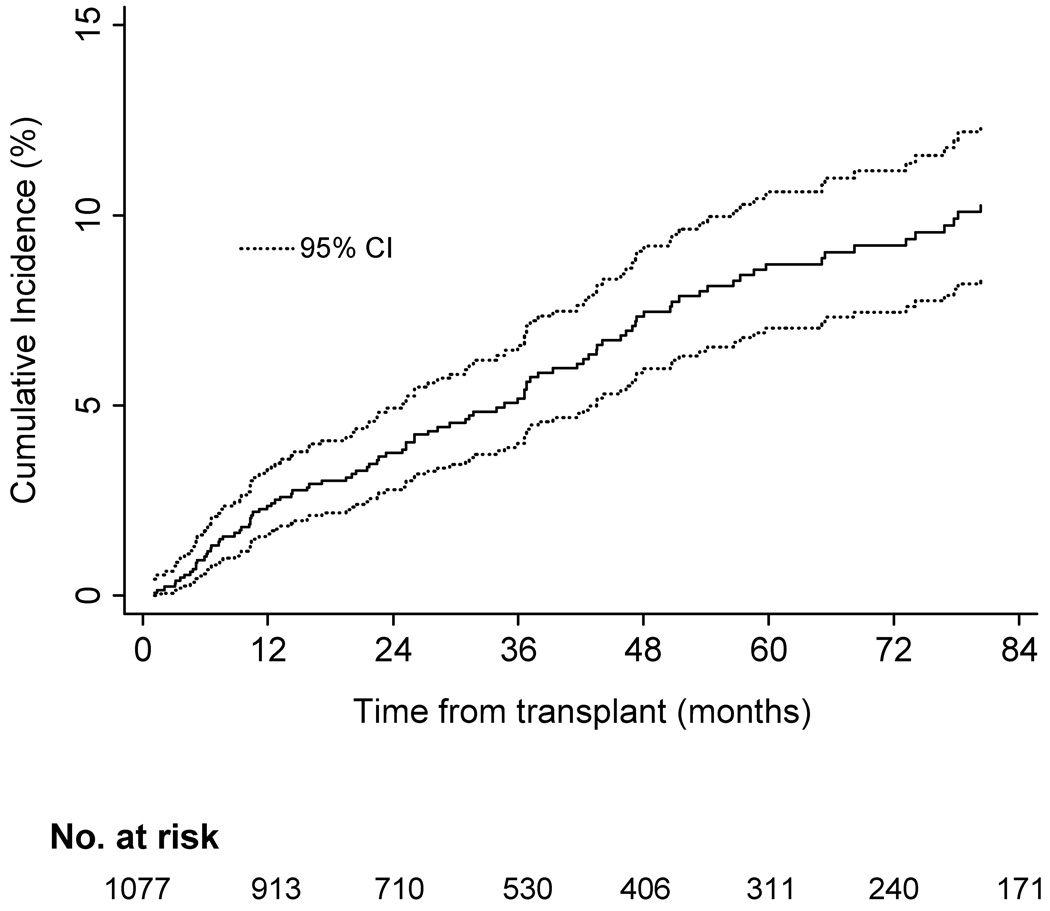
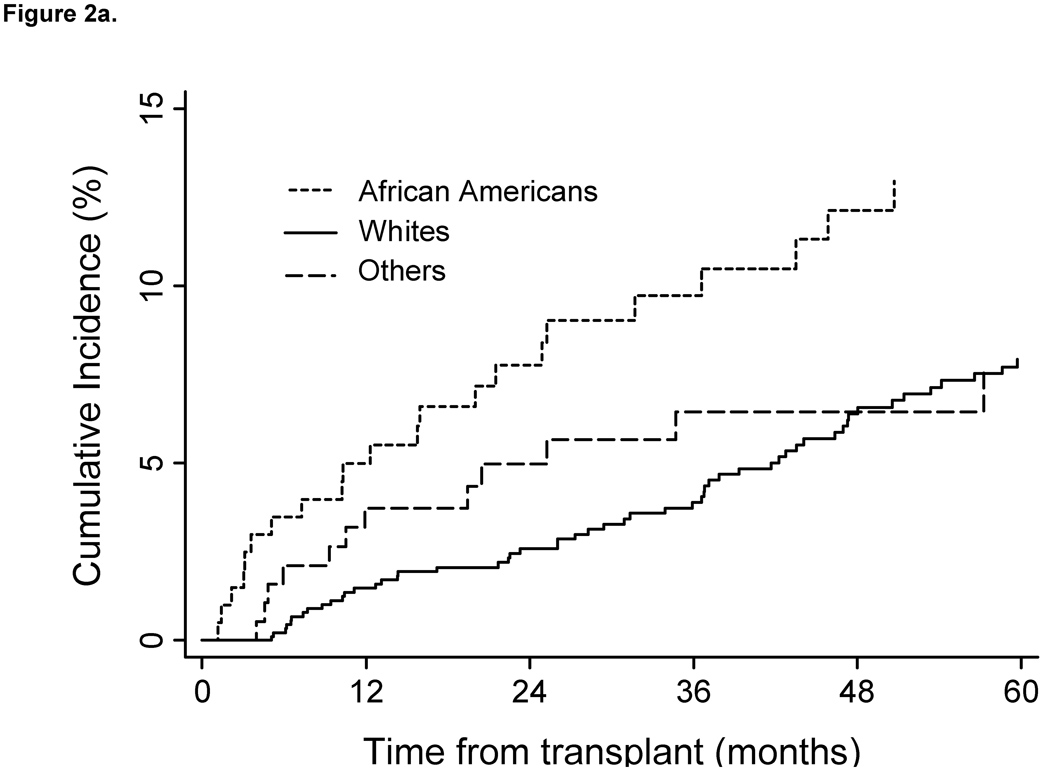
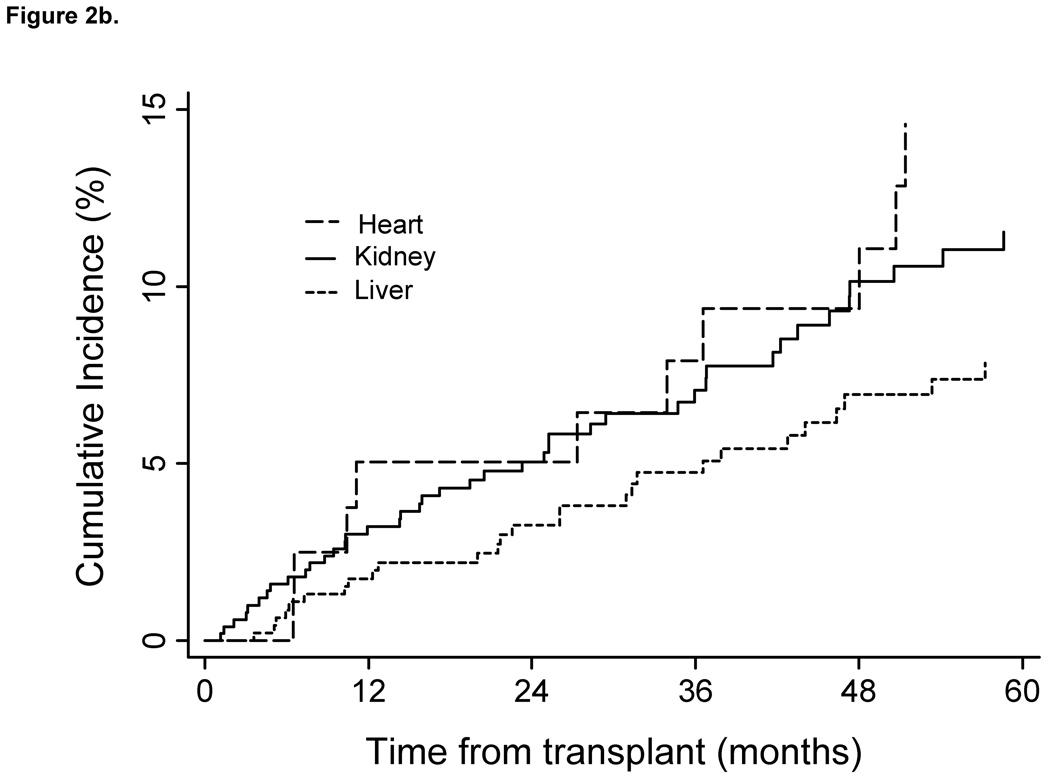
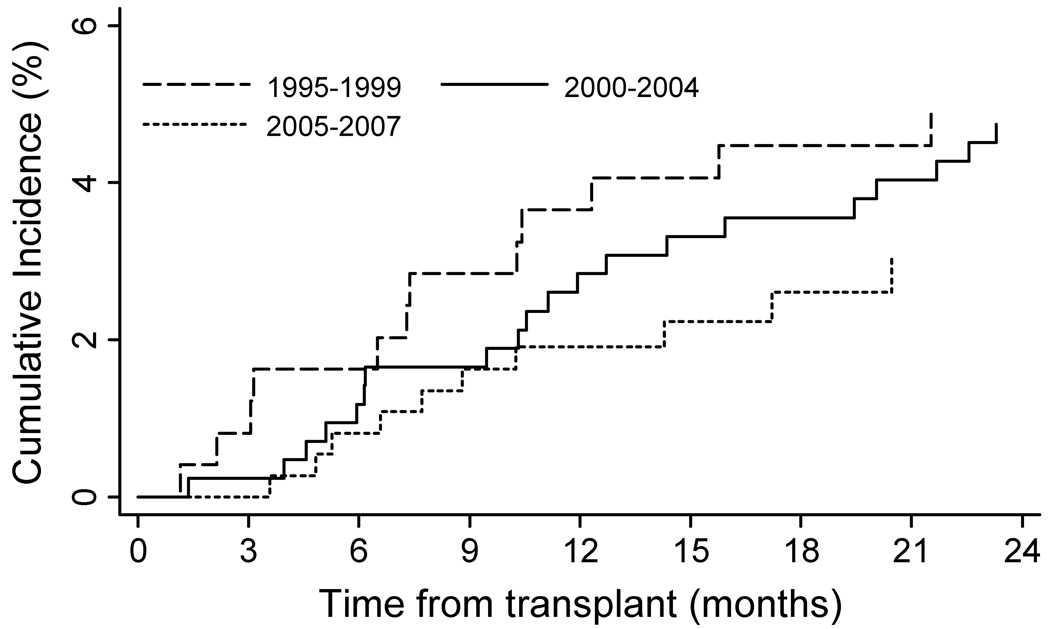
Incidence rates were increased within age strata and declined in patients transplanted during the later calendar years of the cohort also declined (Oct 1996 –1999vs. 2005–2007). Cumulative incidence estimates indicate that patients remained at risk during the entire period follow-up (Fig. 1). As shown in Figure 2A cumulative incidence of HZ also differed by recipient race. Although heart transplants had the highest rates of HZ, no significant difference in incidence was found, based on type of organ transplanted (Fig. 2B). Additionally, no difference in cumulative incidence of HZ was found during the first 2 years post transplant, when compared between calendar years (Fig. 3).
Fig. 1.
Cumulative incidence of herpes zoster post transplant in cohort of VA solid organ transplant recipients from 1996–2007 (n=1077). Cumulative incidence curves censored for lost to follow-up; death and re-transplantation considered competing risks. VA, Veteran’s Affairs Healthcare System; CI, confidence interval.
Fig. 2.
Cumulative incidence of herpes zoster post transplant in cohort of VA solid organ transplant recipients from 1996–2007 by race and organ type (n=1077). Cumulative incidence curves censored for lost to follow-up; death and re-transplantation considered competing risks. (A) Log-rank test between curves showed a significant difference between the three groups. (P=0.023). A log-rank test between curves for Whites and African Americans demonstrated a significant difference between the two groups (P=0.007) Cumulative incidence of herpes zoster by race. (B) A log-rank test between curves showed a significant difference between the three groups (P=0.053). Cumulative incidence of herpes zoster by organ type. VA, Veteran’s Affairs Healthcare System.
Fig. 3.
Cumulative incidence of herpes zoster during the first 2 years post transplant in a cohort of VA solid organ transplant recipients from 1996–2007 by calendar year (n=1077). Cumulative incidence curves censored for lost to follow-up; death and re-transplantation considered competing risks. A log-rank test between curves showed no difference between the three groups (P=0.45). VA, Veteran’s Affairs Healthcare System.
In univariate analyses, the relative hazards of developing HZ in African American transplant recipients were higher when compared to Whites, while other races had similar relative hazards when compared to Whites (Table 3). Other risk factors associated with HZ included organ rejection, timing of transplant (calendar year), and service connection. Patients that developed organ rejection also were at increased hazards for HZ when compared to patients who did not. The type of transplant, patient age, CMV disease, and comorbidity index did not alter the hazard of developing zoster in univariate analyses.
Table 3.
Cox proportional-hazard models for the development of herpes zoster in solid organ transplant recipients 1996 – 2007 (n=1077)
| Variable | Univariate HR (95% CI) |
Multivariable HR (95% CI) |
|---|---|---|
| Age (per 5 yrs) | 1.03 (0.92, 1.15) | 1.13 (1.01, 1.27) |
| Race | ||
| White | 1.0 (referent) | - - |
| African American | 1.92 (1.18, 3.13) | 1.88 (1.12, 3.17) |
| Other | 1.00 (0.54, 1.88) | 1.17 (0.62, 2.21) |
| Type of transplant | ||
| Kidney | 1.0 (referent) | - - |
| Liver | 0.77 (0.49, 1.21) | 1.08 (0.62, 1.89) |
| Heart | 1.69 (0.91, 3.13) | 1.70 (0.87, 3.34) |
| Transplant year1 | 0.60 (0.43, 0.83) | 0.57 (0.40, 0.80) |
| VA status | ||
| Service connected | 1.0 (referent) | - - |
| Non-service connected | 0.57 (0.38, 0.86) | 0.51 (0.33, 0.80) |
| Comorbidity Index2 | ||
| 0 | 1.60 (0.89, 2.88) | 1.28 (0.65, 2.52) |
| 1 | 1.07 (0.55, 2.07) | 1.01 (0.51, 1.98) |
| 2 | 1.50 (0.91, 2.49) | 1.42 (0.81, 2.51) |
| ≥3 | 1.0 (referent) | - - |
| Organ rejection3 | ||
| No | 1.0 (referent) | - - |
| Yes | 1.61 (1.04, 2.51) | 1.42 (0.91, 2.23) |
| CMV3 | ||
| No | 1.0 (referent) | - - |
| Yes | 1.57 (0.83, 2.95) | 1.34 (0.70, 2.54) |
Modeled as group linear term.
Charlson-Deyo Index.
Time-dependent variable in multivariable model.
HR, hazard ratio; CI, confidence interval; VA, US Department of Veteran’s Affairs healthcare system; CMV, cytomegalovirus.
In the multivariable model, the relative hazards of HZ were increased in African Americans compared to Whites, and in those who were older at time of transplant (per 5-year increments) (Table 3). Subjects who were not service connected remained at lower relative hazards for HZ, as did patients transplanted in the latter calendar years of the cohort. In these multivariate analysis, organ rejection, type of organ transplanted, comorbidity index, and CMV disease did not alter hazards of HZ.
Discussion
This study demonstrates a high incidence of HZ in a large multi-center cohort of VA SOT recipients. The overall incidence in this cohort was 22.2 per 1000 patient-years, with African American subjects and heart transplant recipients demonstrating the highest incidence of post-transplant HZ. In a time-dependent model, African Americans, older transplant recipients and those with prior organ rejection also appeared to have higher rates of HZ. Subjects remained at risk throughout the entire post-transplant follow-up period.
To the best of our knowledge, this study is the largest multi-center cohort of SOT recipients to date to assess the incidence of HZ. These data provide important information on long-term risk of HZ following SOT, afford the first assessment of risk in other non-white populations, and present further evidence for an increased risk of HZ in SOT recipients. Other published studies evaluating HZ incidence in this transplant population have been limited to single centers, short follow-up periods, and or a specific type of transplant (kidney, liver, lung, and heart). Corresponding to these varied populations and study methods, the reported incidence of rates of HZ have varied from 1.5% to 16.2% (9–15). Our data are in accordance with the two largest studies published to date (9, 10), which indicate incidence rates anywhere from 2–5 times those seen in the general population (35, 36).
The age-related increase in HZ in older transplant recipients seen in our study is similar in magnitude to that seen in the immunocompetent population, and is likely a reflection of ongoing decline in varicella zoster virus-specific T-cell immunity, in addition to transplant-related immunosuppression (37, 38). These findings are of particular public health importance. Patients aged 60 years and older represent the fastest growing population with end-stage renal disease worldwide (39). Furthermore, the number of new registrants for kidney transplants between the ages of 50 and 64 years has doubled and those aged 65 years and older has nearly tripled (39, 40).
Most studies evaluating HZ incidence in SOT have focused primarily on white recipients. While large databases, such as the United Organ Network for Organ Sharing and the Scientific Registry for Transplant Recipients, would be useful for calculating population based incidence rates, they do not currently collect information on HZ. Our cohort’s racial and ethnic distribution are more consistent with current US census data (41), and provide a longitudinal assessment of HZ incidence in SOT recipients in the US. In addition to assessing incidence in a more heterogeneous population then has previously been possible, the availability of follow-up data reveals important new information on the long-term rate of HZ following SOT.
This is the first study to demonstrate a higher incidence of HZ in African American SOT recipients. The increased incidence seen in African Americans in our study could be caused in part by higher rates of organ rejection in African Americans (42–44), differences in immunosuppressive regimens (45, 46) or metabolism (47–50), or as yet undetermined racial variations in viral immune response, as seen in other viral infections (51–53). The limited but conflicting findings in regards to racial differences in HZ incidence seen in other non-transplant populations (54), also suggest that these findings warrant confirmation in future studies.
Differences in health care utilization could also be a reflection of racial variations in HZ incidence, as African Americans are known to access VA services at higher rates than Whites (55, 56). In addition, the overall incidence rates in our study are most likely an underestimation, as incident cases are expected to be missed in those who sought care at non-VA facilities. Finally, our finding that service connection, a reflection of VA healthcare use, was associated with an increased incidence of HZ is consistent with this hypothesis as improved access would be associated with a higher event capture. These data in African Americans may therefore be a more accurate assessment of HZ risk in SOT, and the rates described in this population will be important to consider in planning future prospective trials.
Heart transplant recipients had a high rate of HZ in our cohort, which is consistent with prior studies (10, 57, 58), and may be a reflection on differences in immunosuppression (59). In contrast, liver transplants are relatively resistant to rejection compared to other organs and generally require less immunosuppression (60). The lower incidence of HZ seen in liver transplant recipients in our cohort and in other studies (10, 13), provides further evidence that intensity of immunosuppression may be important in determining risk of HZ. We can only hypothesize about the decline in HZ incidence in those patients transplanted in more recent years. The decreased incidence seen in latter cohorts is likely in part a result of shorter follow-up and the increasing use of antiviral prophylaxis for CMV, which has been shown to further limit risk of HZ in the first 1–2 years following transplantation (9, 10, 61). Improvements in immunosuppressive regimens, prevention of rejection, and other alterations in antiviral prophylaxis may also play a role in this decline, but will need to be assessed in future studies.
The high penetrance of HZ seen in this study indicates a need for future prevention studies. Current American Society of Transplantation guidelines do not recommend antiviral prophylaxis (62), and to date no formal studies of HZ prevention in SOT have been published. Because daily acyclovir is safe and has been shown to prevent the development of HZ in other immunosuppressed populations (63–65), prospective studies are needed to assess long-term antiviral drug prophylaxis after organ transplantation. Additionally, while the currently available HZ vaccine is contraindicated in SOT recipients (66), future studies are needed to address the safety and efficacy of the live-attenuated virus vaccine in the pre-transplant period. Ongoing studies using a heat-killed HZ vaccine in immunosuppressed populations may provide another option for future prevention (67).
Determining the incidence rate of HZ in SOT recipients is complicated by the diverse demographics of the population, data ascertainment from primarily outpatient records, and the inherent costs of long-term follow-up studies. The VA healthcare system database provided an efficient way to assess the incidence of HZ in large multi-center population of transplant recipients over a 10-year period. However, this analysis has strengths and limitations imposed by these data. It is probable that these data underestimate the true incidence rate in this population, as incident and recurrent cases of HZ in subjects who sought alternate clinical care outside of the VA system were not captured (68). In addition, although rates of post-herpetic neuralgia are similar to rates in other post-transplant populations (9), higher rates reported by others suggest that we may also underestimate the frequency of this complication (10). Because of our use of ICD-9 coding, we could not assess invasive HZ complications or dissemination, both of which may be higher in SOT recipients (69). Furthermore, data on bacterial super-infection need to be assessed in future prospective studies. Most importantly, we could not address critical treatment-related risk factors, such as immunosuppressive regimen and concomitant antiviral therapy, as other studies have done (10, 13). However, our data are strengthened by the size and multi-center nature of the cohort, the length of long-term follow-up, and the racial and ethnic diversity of the VA population. Furthermore, since our incidence rates are likely conservative, they could be useful for developing appropriately powered clinical trials in HZ prevention.
In summary, in this cohort of VA SOT recipients, we found that HZ was a common infectious complication in the post-transplant period. African Americans, subjects receiving heart transplants, and older transplant recipients appeared to have the highest incidence rates of HZ. In addition, in time-dependent analyses, African Americans, subjects who developed organ rejection and older transplant recipients appeared to have higher rates of HZ. The rates of HZ seen during long-term follow-up of this multi-center cohort confirm findings from other large retrospective studies, and provide additional support for future studies in HZ prevention.
Acknowledgements
The authors would like to thank Ted Gooley, PhD and Amelia Margaret, PhD for their assistance with the statistical analyses in this manuscript, and Ernie Ayers, MPTH for his administrative support. This material is based on work supported in part by the Office of Research and Development Cooperative Studies Program, Department of Veterans Affairs and the use of facilities at the VA Puget Sound Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Financial support for this study: This study was supported in part by the Puget Sound VA Epidemiologic Research and Information Center, VA Research & Development, Seattle, WA. S.A.P. is supported by the Joel Meyers Foundation, NIH grant K23HL096831, and an American Society of Blood and Marrow Transplant/Viropharma, Inc. New Investigator Award. M.J.B is supported by NIH grant CA18029 (core C), and K24HL093294. A.W. is supported by K24AI071113. B.A.Y. is supported by NIH grant RO1 DK079745.
Footnotes
None of these agencies were involved in the design, conduct, or reporting of this study.
Presentation of material in submitted manuscript: Presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy /46th Annual Infectious Disease Society of America Conference in Washington, DC, October 25–28, 2008.
References
- 1.Gnann JW, Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347(5):340–346. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 2.Straus SE, Ostrove JM, Inchauspe G, et al. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention; Ann Intern Med; 1988. pp. 221–237. [DOI] [PubMed] [Google Scholar]
- 3.Breuer J. Varicella Zoster virus. In: Zuckerman AJ, Banatvala JE, Pattison JR, Griffiths PD, Schoub BD, editors. Principles and Practice of Clinical virology. 5th edn. Vol. 2B. J Wiley & Sons Ltd; 2004. p. 53. [Google Scholar]
- 4.Liesegang TJ. Varicella zoster viral disease. Mayo Clin Proc. 1999;74(10):983–998. doi: 10.4065/74.10.983. [DOI] [PubMed] [Google Scholar]
- 5.Wareham DW, Breuer J. Herpes zoster. BMJ (Clinical research ed) 2007;334(7605):1211–1215. doi: 10.1136/bmj.39206.571042.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAlister VC, Badovinac K. Transplantation in Canada: report of the Canadian Organ Replacement Register. Transplant Proc. 2003;35(7):2428–2430. doi: 10.1016/j.transproceed.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. [Accessed June 15, 2009];Organ Procurement and Transplantation Network. 2009 June 5; http://optn.transplant.hrsa.gov/latestData/rptData.asp.
- 8.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 9.Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10(4):260–268. doi: 10.1111/j.1399-3062.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- 10.Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant. 2004;4(1):108–115. doi: 10.1046/j.1600-6143.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 11.Herrero JI, Quiroga J, Sangro B, et al. Herpes zoster after liver transplantation: incidence, risk factors, and complications. Liver Transpl. 2004;10(9):1140–1143. doi: 10.1002/lt.20219. [DOI] [PubMed] [Google Scholar]
- 12.Karakayali H, Emiroglu R, Arslan G, Bilgin N, Haberal M. Major infectious complications after kidney transplantation. Transplant Proc. 2001;33(1–2):1816–1817. doi: 10.1016/s0041-1345(00)02694-4. [DOI] [PubMed] [Google Scholar]
- 13.Levitsky J, Kalil A, Meza JL, Hurst GE, Freifeld A. Herpes zoster infection after liver transplantation: a case-control study. Liver Transpl. 2005;11(3):320–325. doi: 10.1002/lt.20356. [DOI] [PubMed] [Google Scholar]
- 14.Manuel O, Kumar D, Singer LG, Cobos I, Humar A. Incidence and clinical characteristics of herpes zoster after lung transplantation. J Heart Lung Transplant. 2008;27(1):11–16. doi: 10.1016/j.healun.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Oberbauer R, Kreis H, Johnson RW, et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation. 2003;76(2):364–370. doi: 10.1097/01.TP.0000074360.62032.39. [DOI] [PubMed] [Google Scholar]
- 16.Triemer HL, Pearson TC, Odom KL, Larsen CP. Analysis of a single-center experience with mycophenolate mofetil based immunosuppression in renal transplantation. Clin Transplant. 2000;14(4 Pt 2):413–420. doi: 10.1034/j.1399-0012.2000.14041002.x. [DOI] [PubMed] [Google Scholar]
- 17.Office of Human Resources and Administration. 2008 Organizational Briefing Book. [on July 13, 2008];Department of Veteran's Affairs. 2008 May; Downloaded from http://www.va.gov/ofcadmin/docs/vaorgbb.pdf.
- 18.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3 Suppl):92S–123S. doi: 10.1177/1077558703256726. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211–1215. doi: 10.1161/01.STR.0000259622.78616.ea. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227–1232. doi: 10.1345/aph.1M017. [DOI] [PubMed] [Google Scholar]
- 22.Borowsky SJ, Cowper DC. Dual use of VA and non-VA primary care. J Gen Intern Med. 1999;14(5):274–280. doi: 10.1046/j.1525-1497.1999.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolinsky FD, Coe RM, Mosely RR, 2nd, Homan SM. Veterans' and nonveterans' use of health services. A comparative analysis. Medical Care. 1985;23(12):1358–1371. doi: 10.1097/00005650-198512000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Young BA, Maynard C, Reiber G, Boyko EJ. Effects of ethnicity and nephropathy on lower-extremity amputation risk among diabetic veterans. Diabetes Care. 2003;26(2):495–501. doi: 10.2337/diacare.26.2.495. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Geneva: WHO; Manual of international statistical classification of diseases, injuries and causes of death, based on the recommendations of the Ninth Revision Conference, 1975. 1997
- 26.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155(15):1605–1609. [PubMed] [Google Scholar]
- 27.White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics. 2009;27(9):781–792. doi: 10.2165/11317560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 30.Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Managed Care. 2002;8(1):37–43. [PubMed] [Google Scholar]
- 31.Kashner TM. Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Medical Care. 1998;36(9):1324–1336. doi: 10.1097/00005650-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 32.McDonald JR, Zeringue AL, Caplan L, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009 doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 34.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Statistics in medicine. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 35.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 37.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30(6):582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 38.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142(2):291–293. [PubMed] [Google Scholar]
- 39.Sener A, Schweitzer EJ, Munivenkatappa R, et al. Deceased-donor renal transplantation in the geriatric population demonstrates equal graft survival compared with younger recipients. Transplantation. 2009;87(10):1549–1554. doi: 10.1097/TP.0b013e3181a4b67d. [DOI] [PubMed] [Google Scholar]
- 40.2007 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997–2006. Richmond, VA: United Network for Organ Sharing; Department of Health and Human Services, Health Resources and Services Administration, Health Systems Bureau, Division of Transplantation, Rockville, MD.
- 41.US Census Bureau Quick Facts. United States Census Bureau. [on July 12, 2008];2008 Accessed at http://quickfacts.census.gov/qfd/states/00000.html.
- 42.Tang H, Chelamcharla M, Baird BC, Shihab FS, Koford JK, Goldfarb-Rumyantzev AS. Factors affecting kidney-transplant outcome in recipients with lupus nephritis. Clin Transplant. 2008;22(3):263–272. doi: 10.1111/j.1399-0012.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 43.Hong JC, Kosari K, Benjamin E, et al. Does race influence outcomes after primary liver transplantation? A 23-year experience with 2,700 patients. J Am Coll Surg. 2008;206(5):1009–1016. doi: 10.1016/j.jamcollsurg.2007.12.019. discussion 1016–1008. [DOI] [PubMed] [Google Scholar]
- 44.Cohen O, De La Zerda D, Beygui RE, Hekmat D, Laks H. Ethnicity as a predictor of graft longevity and recipient mortality in heart transplantation. Transplant Proc. 2007;39(10):3297–3302. doi: 10.1016/j.transproceed.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 45.Ahsan N, Hricik D, Matas A, et al. Steroid Withdrawal Study Group. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil--a prospective randomized study. Transplantation. 1999;68(12):1865–1874. doi: 10.1097/00007890-199912270-00009. [DOI] [PubMed] [Google Scholar]
- 46.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 47.Dirks NL, Huth B, Yates CR, Meibohm B. Pharmacokinetics of immunosuppressants: a perspective on ethnic differences. Int J Clinl Pharmacol Therapeutics. 2004;42(12):701–718. doi: 10.5414/cpp42701. [DOI] [PubMed] [Google Scholar]
- 48.Macphee IA, Fredericks S, Mohamed M, et al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79(4):499–502. doi: 10.1097/01.tp.0000151766.73249.12. [DOI] [PubMed] [Google Scholar]
- 49.Bernard O, Tojcic J, Journault K, Perusse L, Guillemette C. Influence of nonsynonymous polymorphisms of UGT1A8 and UGT2B7 metabolizing enzymes on the formation of phenolic and acyl glucuronides of mycophenolic acid. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(9):1539–1545. doi: 10.1124/dmd.106.010553. [DOI] [PubMed] [Google Scholar]
- 50.Tornatore KM, Reed KA, Venuto RC. Racial differences in the pharmacokinetics of methylprednisolone in black and white renal transplant recipients. Pharmacotherapy. 1993;13(5):481–486. [PubMed] [Google Scholar]
- 51.Rhodes SL, Erlich H, Im KA, et al. Associations between the human MHC and sustained virologic response in the treatment of chronic hepatitis C virus infection. Genes Immunity. 2008;9(4):328–333. doi: 10.1038/gene.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen HR, Weston SJ, Im K, et al. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology. 2007;46(2):350–358. doi: 10.1002/hep.21714. [DOI] [PubMed] [Google Scholar]
- 53.Thio CL, Carrington M, Marti D, et al. Class II HLA alleles and hepatitis B virus persistence in African Americans. J Infect Dis. 1999;179(4):1004–1006. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 54.Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171(3):701–704. doi: 10.1093/infdis/171.3.701. [DOI] [PubMed] [Google Scholar]
- 55.Saha S, Freeman M, Toure J, Tippens KM, Weeks C. Racial and Ethnic Disparities in the VA Healthcare System: A Systematic Review. [on March 10, 2009];2007 June; Accessed from http://www.hsrd.research.va.gov/publications/esp/RacialDisparities-2007.pdf. [PubMed]
- 56.Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654–671. doi: 10.1007/s11606-008-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabezon Ruiz S, Cisneros JM, Lage Galle E, et al. Characteristics and repercussion of varicella-zoster virus infection in cardiac transplant. Transplant Proc. 2003;35(5):2004–2005. doi: 10.1016/s0041-1345(03)00652-3. [DOI] [PubMed] [Google Scholar]
- 58.Hsu RB, Fang CT, Chang SC, et al. Infectious complications after heart transplantation in Chinese recipients. Am J Transplant. 2005;5(8):2011–2016. doi: 10.1111/j.1600-6143.2005.00951.x. [DOI] [PubMed] [Google Scholar]
- 59.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 60.Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut. 2009;58(3):452–463. doi: 10.1136/gut.2008.163527. [DOI] [PubMed] [Google Scholar]
- 61.Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV. Herpesvirus infections in solid organ transplant patients at high risk of primary cytomegalovirus disease. J Infect Dis. 2005;192(8):1331–1339. doi: 10.1086/466529. [DOI] [PubMed] [Google Scholar]
- 62.Pergam SA, Limaye AP. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S108–S115. doi: 10.1111/j.1600-6143.2009.02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110(8):3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 64.Asano-Mori Y, Kanda Y, Oshima K, et al. Long-term ultra-low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2008;83(6):472–476. doi: 10.1002/ajh.21152. [DOI] [PubMed] [Google Scholar]
- 65.Boeckh M. Prevention of VZV infection in immunosuppressed patients using antiviral agents. Herpes. 2006;13(3):60–65. [PubMed] [Google Scholar]
- 66.Zostavax® [package insert] Whitehouse Station, NJ: Merck & Co., Inc.; 2009. Jul, [Google Scholar]
- 67.Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347(1):26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 68.Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Medical Care. 2005;43(5):453–460. doi: 10.1097/01.mlr.0000160377.82164.d3. [DOI] [PubMed] [Google Scholar]
- 69.Fuks L, Shitrit D, Fox BD, et al. Herpes zoster after lung transplantation: incidence, timing, and outcome. Ann Thorac Surg. 2009;87(2):423–426. doi: 10.1016/j.athoracsur.2008.11.004. [DOI] [PubMed] [Google Scholar]