Abstract
The aim of this study was to investigate the possibility of using pectinate micro/nanoparticles as gene delivery systems. Pectinate micro/nanoparticles were produced by ionotropic gelation. Various factors were studied for their effects on the preparation of pectinate micro/nanoparticles: the pH of the pectin solution, the ratio of pectin to the cation, the concentration of pectin and the cation, and the type of cation (calcium ions, magnesium ions and manganese ions). After the preparation, the size and charge of the pectin micro/nanoparticles and their DNA incorporation efficiency were evaluated. The results showed that the particle sizes decreased with the decreased concentrations of pectin and cation. The type of cations affected the particle size. Sizes of calcium pectinate particles were larger than those of magnesium pectinate and manganese pectinate particles. The DNA loading efficiency showed that Ca-pectinate nanoparticles could entrap DNA up to 0.05 mg when the weight ratio of pectin:CaCl2:DNA was 0.2:1:0.05. However, Mg-pectinate could entrap only 0.01 mg DNA when the weight ratio of pectin:MgCl2:DNA was 1:100:0.01 The transfection efficiency of both Ca-pectinate and Mg-pectinate nanoparticles yielded relatively low levels of green fluorescent protein expression and low cytotoxicity in Huh7 cells. Given the negligible cytotoxic effects, these pectinate micro/nanoparticles can be considered as potential candidates for use as safe gene delivery carriers.
Key words: gene delivery, Huh7 cells, micro/nanoparticle, pectin
INTRODUCTION
Gene therapy has become a promising strategy for the treatment of many inheritable or acquired diseases that are currently considered incurable. The main objective in gene therapy is successful in vivo transfer of the genetic materials to the targeted tissues (1–2). However, naked therapeutic genes are rapidly degraded by nucleases and show poor cellular uptake, so the development of safe and efficient gene carriers is one of the prerequisites for the success of gene therapy (3). The development of new delivery systems for the administration of gene therapeutics is a field of great interest. Gene delivery has been regarded as a powerful tool for curing disease by replacing defective genes, substituting missing genes or silencing unwanted gene expression. One approach is a non-viral delivery system based on supramolecular assembly. Cationic lipids (4–6) and cationic polymers (7–10) have been employed as non-viral gene transfer agents. These cationic substances form complexes with anionic DNA by electrostatic interaction. The resultant cationic DNA complexes were taken up by cells through electrostatic interaction because the cell surface is negatively charged. Transfection efficiencies of these complexes have been investigated in vivo and in vitro. However, before putting these complexes to practical use, there are several problems to overcome: insufficient transfection efficiency, strong cytotoxicity, and inhibition by serum. In order to increase the stability of the carriers, nanoparticles were prepared. Nanoparticles have received considerable attention for the delivery of macromolecular drugs, such as peptides, proteins, and genes, due to their ability to protect the labile drugs from enzymatic degradation. In addition, the endocytic uptake of nanoparticles can help the drug cargo to breach the permeability barriers posed by absorption epithelia (11). Biodegradable and biocompatible polymers are suitable for human use and can be prepared as particle complexes of various sizes. Particles with suitable sizes and charges can enter mammalian cells by several routes, such as pinocytosis, phagocytosis, receptor-mediated uptake, etc. (12), and this may improve the chances of cellular entry.
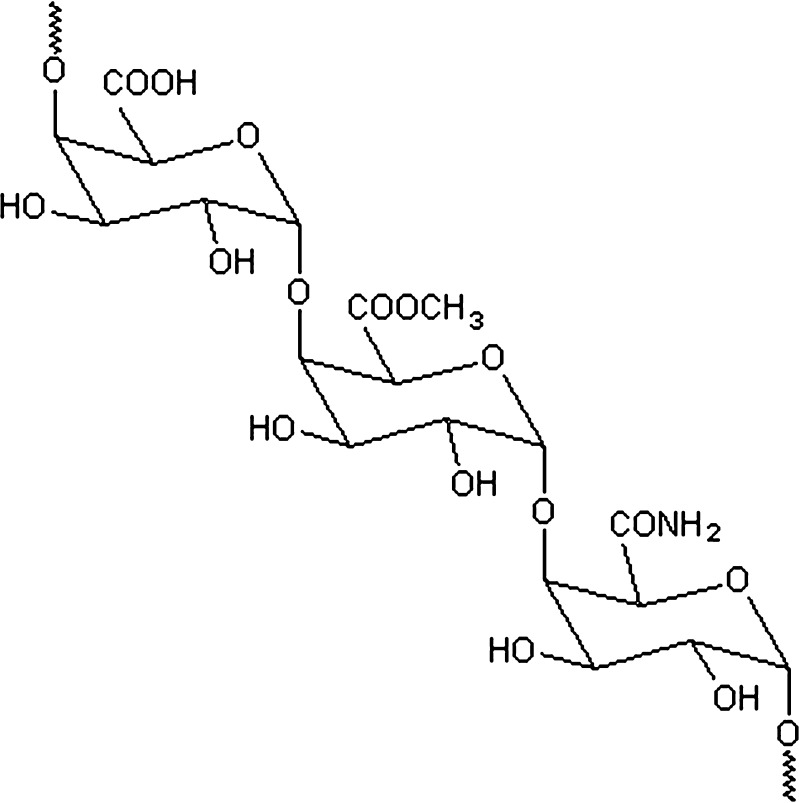
Pectins are anionic, soluble, non-starch polysaccharides extracted from the primary cell walls of plants. Pectins, a heterogeneous complex polysaccharide of linear 1,4-linked α-d-galacturonic acid (Fig. 1), are used as gelling and thickening agents in food industries (13). In recent years, the polymer has also been explored as a pharmaceutical excipient (14). The functional properties of pectin are determined by the percentage of carboxyl groups that have been esterified or amidated, denoted as the degree of esterification (DE) and degree of amidation (DA), respectively. Amidated low-methoxy pectin (DE < 50%) forms more rigid gels by the action of calcium, which cross-links the galacturonic acid chains, than does only methoxy pectin. The lower the DE, the more sensitive the pectin to calcium (13). Several researchers successfully incorporated protein or peptide into calcium pectinate beads for a colonic delivery system (15–19). Cheng et al. (20) has succeeded in formulating insulin-loaded calcium pectinate nanoparticles by adapting an ionotropic gelation method reported for the manufacture of calcium pectinate beads. This method is advantageous by virtue of being a simple method that does not involve expensive equipment, harsh processing conditions, or organic solvent systems. Recently, alginate-based nanoparticles were reported to protect an associated oligonucleotide and, as a potential carriers, to enhance the oral bioavailability of antisense therapeutics (21). No data on use of pectinate micro/nanoparticles as a non-viral vector is available. Since the size of micro/nanoparticles is essential for efficient gene expression, the main objective of this study was to establish a micro/nanoparticulate system produced by ionotropic gelation between a cation and pectin, and to evaluate their potential as DNA carriers. The physical and morphological properties of nanoparticles were investigated in accordance with formulation parameters. Their transfection efficiencies and cytotoxicity in human hepatocellular carcinoma cells (Huh7 cells) were evaluated.
Fig. 1.
Chemical structure of amidated pectin
MATERIALS AND METHODS
Materials
Amidated low-methoxy (LM) pectins (molecular weight: 150 kDa) with 27–32% degree of esterification (DE) and 18–23% degree of amidation (DA; Pectin Amid CU 020) were the generous gifts of Herbstreith and Fox KG, Germany. Calcium chloride, magnesium chloride, manganese chloride and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Chemical Co., USA. Agarose was purchased from ISC Bioexpress, USA. High-molecular-weight polyethylenimine (25 kDa), was purchased from Aldrich, Germany. Dulbecco’s modified Eagle’s medium (DMEM), Trypsin-EDTA, penicillin-streptomycin antibiotics and fetal bovine serum (FBS) were obtained from Gibco-Invitrogen, USA. All other chemicals were of cell-culture and molecular-biology quality.
The pEGFP-C2 Plasmid DNA, encoding enhanced green fluorescent protein (EGFP), was obtained from Clontech, USA. The λDNA/HindIII were obtained from Promega, USA. The pEGFP-C2 plasmid was extracted by the plasmid Maxiprep protocol. The purity and integrity of the plasmid were assessed by UV spectrophotometer (A260/A280 ratio) and electrophoresis on a 0.8% agarose gel using λDNA/HindIII as a DNA marker.
Methods
Preparation of Pectinate Micro/Nanoparticles
Pectinate micro/nanoparticles were prepared by an ionic gelation procedure. Briefly, pectin was dissolved in distilled water to a concentration ranging from 10 to 0.1 mg/ml. The divalent cation (CaCl2, MgCl2 or MnCl2) was dissolved in distilled water to a concentration ranging from 10 to 0.1 mg/mL. Nanoparticles were prepared by drop-wise addition of pectin solution to a divalent cation (CaCl2, MgCl2 and MnCl2) solution while the solution was stirred at 25°C. Stirring continued for another 20 min, and then the resultant particles were collected for size determination.
Preparation of Plasmid DNA-Loaded Pectinate Micro/Nanoparticles
The effect of formulation on the plasmid DNA-loaded pectinate micro/nanoparticles was evaluated. Plasmid DNA (0.01–0.5 mg) was premixed with 0.2 ml of pectin solution. The desired pH was obtained by adding 0.25 M HCl to the pectin solution to a final pH of 4; then pectin solution was added to 2 ml of divalent cation (CaCl2 or MgCl2) solution while the solution was stirred at 25°C. Stirring continued for another 20 min, and then the resultant particles were collected and evaluated for size determination, morphology and plasmid DNA entrapment efficiency.
Characterization of the plasmid DNA-loaded pectinate micro/nanoparticles
Particle Size and Zeta-Potential Measurements
The mean particle size and zeta potential of the plasmid DNA-loaded pectinate micro/nanoparticles were determined in triplicate at 25°C by photon correlation spectroscopy (PCS) using the Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The pectinate micro/nanoparticulate samples were diluted with distilled water that had been filtered through a 0.22-μm membrane filter.
Morphology
The morphology of the plasmid DNA-loaded pectinate nanoparticles was investigated by transmission electron micrograph. Briefly, three percentage solution of formvar was prepared in spectroscopic-grade chloroform. Then, one drop of pectinate nanoparticulate sample solution was put on a formvar-coated carbon ultra-thin grid and air-dried. The dried grid was then examined under transmission electron microscope (JEOL JEM1230, Tokyo, Japan).
Plasmid DNA Association Capacity
The pDNA association capacity of the micro/nanoparticles was evaluated after separation of the micro/nanoparticles by centrifugation of the nanoparticle suspension at 8,000 rpm for 30 min. The amount of free pDNA in the supernatant was determined in triplicate by GeneRay UV photometer (Biometra® λ260/280 nm). The pDNA association capacity was calculated as previously reported (21) as presented in Eq. 1.
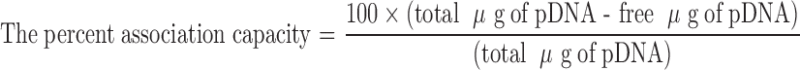 |
1 |
The pDNA association capacity was confirmed by electrophoresis on a 1.0% agarose gel. Briefly, agarose gels were prepared with 1% agarose solution in tris acetate-EDTA buffer, pH 8.0 (TAE buffer). The electrophoresis was carried out for 60 min at 100 V. Fifteen microlitres of DNA-loaded micro/nanoparticles were loaded into each well. UV transillumination of the gel was employed with ethidium bromide (0.5 μg/ml) to visualize the DNA.
In Vitro Gene Expression
Huh7 cells were seeded for 24 h into 24-well plates at a density of 5 × 10 cells/cm2 in 1 ml of growth medium (DMEM containing 10% FBS, supplemented with 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin). Prior to transfection, the medium was removed and the cells were rinsed with phosphate-buffered saline (PBS, pH 7.4), and then supplied with 500 μl of fresh culture medium without FBS. Five hundred microlitres of the DNA-loaded pectinate nanoparticles containing 1 μg of pEGFP-C2 plasmid DNA was incubated with the cells for 24 h at 37°C under 5% CO2 atmosphere. Non-treated cells and cells transfected with naked plasmid used as controls were incubated at the same time. After 24 h, the transfection media were removed and the cells were further cultivated with 1 mL of culture medium for 3 days at 37°C under 5% CO2 atmosphere. All transfection experiments were performed in triplicate.
For fluorescence assay of transfection, the cells were directly viewed under a fluorescence microscope (Model: GFP-B; wavelengths: excitation filter 480/40 and emission filter 535/50) as shown in Fig. 3. The transfection efficiency was calculated by transfected-cells (green color) per area of a 24-well tissue culture plate (1.9 cm2).
Fig. 3.
TEM micrographs of a pDNA-loaded Ca-pectinate (pectin/CaCl2/pDNA = 0.2:1:0.05); b pDNA-loaded Mg-pectinate (pectin/MgCl2/pDNA = 1:100:0.01). Magnification: ×100,000
Cytotoxicity of Plasmid DNA-loaded Pectinate Micro/Nanoparticles
Evaluation of plasmid DNA-loaded pectinate micro/nanoparticles’ cytotoxicity was performed by MTT assay (22). Huh7 cells were seeded at a cell density of 8,000 cells/well in 96-well plates with 100 μl medium and incubated for 24 h prior to experiments. Two hundred fifty microlitres of the plasmid DNA-loaded pectinate micro/nanoparticles with 0.2 μg/well DNA was added and incubated with the cells for 24 h at 37°C under 5% CO2 atmosphere. After 24 h, the mixture was replaced with fresh cell culture medium and the cells were incubated for an additional 24 h at 37°C. After incubation, twenty microlitres of MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, 5 mg/ml) was added to each well and the incubation was continued for 4 h. Then, the medium was removed, the cells were rinsed with PBS of pH 7.4, and formazan crystals formed in the living cells were dissolved in 100 μl DMSO per well. Relative viability (%) was calculated based on absorbance at 550 nm using a microplate reader (340 ATTC; SLT Lab Instruments, Salzburg, Austria). Viability of non-treated control cells was arbitrarily defined as 100%.
RESULTS AND DISCUSSION
Preparation of Pectinate Microparticles
Pectin meets the necessary requirements for a matrix to be used for drug delivery applications; it is biocompatible, biodegradable, non-toxic and easily processed. The preparation strategy for pectinate nanoparticles containing plasmid DNA depends on the gel properties of pectin and its ability to form stable ionotropic gelation. When the aqueous solution of pectin was dropped into divalent cation solution (CaCl2, MgCl2 or MnCl2), pectinate particles were instantaneously produced by ionotropic gelation. In this process, intermolecular cross-links were formed between the negatively charged carboxyl groups of pectin and the positively charged divalent cations, as previously described in the “egg-box Model” (23). Preliminary experiments showed that gelation of pectin were induced by adding divalent cations (CaCl2, MgCl2 and MnCl2). Moreover, these cations have been reported as a factor not only increasing transformation efficiency of bacterial cells with plasmids (24–27) but also inducing transfection of mammalian cells (28–29). To investigate the conditions for micro/nanoparticle formation, systems that were able to produce gel aggregates were identified depending on the concentration of pectin and divalent cations. To determine the concentration limit in micro/nanoparticle formation of pectinate in the presence of divalent cations, several systems with decreasing pectin and divalent cation concentrations were prepared. In each formula, 0.2 ml pectin solutions were added drop-wise to 2 ml of divalent cation. Table I shows the final composition of each system and its corresponding macroscopic evaluation. As shown in Table I, with the pectin solution at a lower concentration, smaller particles were formed in all systems regardless of divalent cation choice. Mg- and Mn-pectinate were smaller in size than Ca-pectinate when formulated using the same amount and ratio of pectin/divalent cations. Ca- and Mg-pectinate formed spherical particle gel beads, whereas Mn-pectinate particle formed rod shapes. The effect of the divalent cation concentration on the size and morphology of the particle revealed that lower divalent cation concentrations yielded smaller sizes and more spherical shapes for all divalent cations.
Table I.
Macroscopic Evaluation of Pectinate Particles Formulated with 0.2 ml of Pectin at a Concentration of 1, 6 and 10 mg/ml, with 2 ml Divalent Cation (CaCl2, MgCl2 and MnCl2) at a Concentration of 8 mg/ml
| Formula Composition | Macroscopic Evaluation of Pectinate Particles | ||||
|---|---|---|---|---|---|
| Pectin conc. (mg/ml) | Divalent cation conc (mg/ml) | Pectin: Cation (w/w) | Ca-Pectinate | Mg-Pectinate | Mn-Pectinate |
| 1 | 8 | 0.0125:1 | Small spherical particles | Small spherical particles | Small particles |
| 6 | 8 | 0.075:1 | Small spherical gel beads | Small spherical gel beads | Small rod gel beads |
| 10 | 8 | 0.125:1 | Small spherical gel beads | Small spherical gel beads | Large rod gel beads |
| 10 | 1 | 1:1 | Small spherical particles | Small spherical particles | Small particles |
| 10 | 6 | 0.17:1 | Spherical gel beads | Small spherical particles | Large rod gel beads |
| 10 | 10 | 0.1:1 | Spherical gel beads | Spherical gel beads | Large rod gel beads |
Dissolved pectin is negatively charged at neutral pH and approaches zero charge at low pH. The pKa-value of pectin is about 3.5. The pH of the formulation could therefore affect the degree of ionization of the pectin molecule and its electrostatic interaction with cations. The zeta potential for 0.1% pectin solution increased from −4 to −54 mV as pH increased from 2 to 5.5; the highest zeta potential was obtained at pH 5.5. High methoxy pectin is known to rapidly degrade at high pH (≥5) (20). The rate of deesterification is faster than the rate of depolymerization. Maximal stability is found at pH 4. At low pH-values and elevated temperatures, degradation due to hydrolysis of glycosidic links is observed. De-esterification is also favored in low pH. Mild conditions can be chosen, so extensive deesterification can be achieved with only little depolymerization. In both cases, highly esterified pectins are more prone to depolymerization than are low methoxy pectins. Depolymerization at low pH-values is a hydrolysis reaction. Depolymerization at alkaline conditions is a beta-elimination reaction. Glycosidic bonds to O-4 of an esterified galacturonic acid subunit. Consequently, the rate of beta-elimination is almost proportional to the amount of remaining methyl ester groups and slows down as they are saponified. Low methoxy pectins show a somewhat better stability as these conditions (13). The effect of pectin solution pH on the characteristics of pectinate particle was evaluated. To prepare pectinate particles at different pHs (2 and 4), pectin solution and divalent cations at the concentration of 10 mg/mL were used. Table II, with its macroscopic evaluation of the particles, showed that in all divalent cations (Ca2+, Mg2+, and Mn2+), the low-pH (2) pectin solution yielded larger sizes than the high-pH (4). This might be attributable to a higher degree of ionization of the pectin molecule at pH 4, which would allow for greater binding between the negatively charged pectin and the positively charged cations, thereby accounting for a smaller size. As a compromise, a maximum pH of 4 was selected; this would give the pectin solution zeta potential values of about −30 mV without inducing considerable depolymerization of the pectin molecules and giving small pectin-cation particles.
Table II.
Macroscopic Evaluation of Pectinate Particles Formulated with 10 mg/ml of Pectin Solution Adjusted to pH 2.0 ± 0.1 and 4.0 ± 0.1, with 10 mg/ml of Divalent Cation (CaCl2, MgCl2 and MnCl2)
| Divalent Cation | Macroscopic Evaluation of Pectinate Particles | |
|---|---|---|
| Pectin sol. pH 2.0 ± 0.1 | Pectin sol. pH 4.0 ± 0.1 | |
| CaCl2 | Spherical gel beads | Small spherical particles |
| MgCl2 | Spherical particles | Small spherical particles |
| MnCl2 | Large rod gel beads | Small rod particles |
Preparation of Pectinate Nanoparticles
In order to prepare the submicron or nano-size pectinate particle for carrier DNA, the pectin solutions were adjusted to pH 4 and the 1- to 0.1-mg/ml concentrations were mixed. Mn-pectinate formed rod shapes and could not form the spherical particles. Therefore, Mn-pectinate might not be suitable for further study. CaCl2 and MgCl2 were selected as divalent cations for preparation of pectinate nanoparticles. The CaCl2 and MgCl2 concentration ranged from 10 to 0.1 mg/ml. Table III shows the particle size and zeta potential of the particles prepared with CaCl2 and MgCl2. Small particles were obtained for the low concentration of pectin and divalent cations. Increasing the solid content resulted in an increase in particle size. Mg-pectinate yielded smaller sizes than Ca-pectinate. The formula that resulted in the smallest particle and a neutral charge was 0.1 mg/ml pectin solution with 0.1 mg/ml CaCl2 solution at the weight ratio of 0.2:1 (F1). In the case of Mg-pectinate, the best formula was 0.5 mg/ml pectin solution with 10 mg/ml MgCl2 solution at the weight ratio of 0.01:1 (F2). These 2 formulae (F1 and F2) were selected for DNA-loaded pectinate submicron particles.
Table III.
Particle Sizes and Zeta Potential of Calcium Pectinate Particles and Magnesium Pectinate Particles
| Formula composition | Ca-Pectinate Particles | Mg-Pectinate Particles | ||||
|---|---|---|---|---|---|---|
| Pectin Conc. (mg/ml) | Divalent Cation Conc (mg/ml) | Pectin/Cation (w/w) | Mean diameter (nm ± SD) | Zeta potential (mV ± SD) | Mean diameter (nm ± SD) | Zeta potential (mV ± SD) |
| 1.0 | 10 | 0.02:1 | >1,000 | NM | 686.1 ± 22 | −7.45 ± 0.4 |
| 1.0 | 6 | 0.03:1 | >1,000 | NM | 655.2 ± 45 | −8.11 ± 1.2 |
| 1.0 | 1 | 0.2:1 | >1,000 | NM | 554.3 ± 12 | −7.91 ± 1.4 |
| 1.0 | 0.5 | 0.4:1 | >1,000 | NM | 314.0 ± 22 | −7.76 ± 0.6 |
| 1.0 | 0.1 | 2:1 | 919.1 ± 23 | −13.3 ± 0.3 | 604.1 ± 51 | −13.3 ± 0.5 |
| 0.5 | 10 | 0.01:1 | >1,000 | NM | 562.1 ± 24b | −1.51 ± 0.1 |
| 0.5 | 6 | 0.02:1 | >1,000 | NM | 611.5 ± 12 | −3.77 ± 0.2 |
| 0.5 | 1 | 0.1:1 | >1,000 | NM | 766.5 ± 44 | −7.41 ± 1.4 |
| 0.5 | 0.5 | 0.2:1 | >1,000 | NM | 764.8 ± 15 | −8.34 ± 1.2 |
| 0.5 | 0.1 | 1:1 | 955.2 ± 45 | −10.4 ± 0.8 | 655.3 ± 32 | −7.24 ± 0.7 |
| 0.1 | 10 | 0.002:1 | >1,000 | NM | 955.3 ± 38 | −9.05 ± 0.4 |
| 0.1 | 6 | 0.003:1 | >1,000 | NM | 877.4 ± 27 | −8.31 ± 1.1 |
| 0.1 | 1 | 0.02:1 | >1,000 | NM | 655.2 ± 31 | −3.16 ± 1.3 |
| 0.1 | 0.5 | 0.04:1 | >1,000 | NM | 558.1 ± 41 | −3.98 ± 0.1 |
| 0.1 | 0.1 | 0.2:1 | 904.5 ± 44a | −6.46 ± 0.5 | 656.3 ± 29 | −5.45 ± 0.7 |
NM Not measurement
aIndicated F1 (0.1 mg/ml pectin solution: 0.1 mg/ml CaCl2 solution at the weight ratio of 0.2:1)
bIndicated F2 (0.5 mg/ml pectin solution: 10 mg/ml MgCl2 solution at the weight ratio of 0.01:1)
Plasmid DNA-loaded Pectinate Nanoparticles
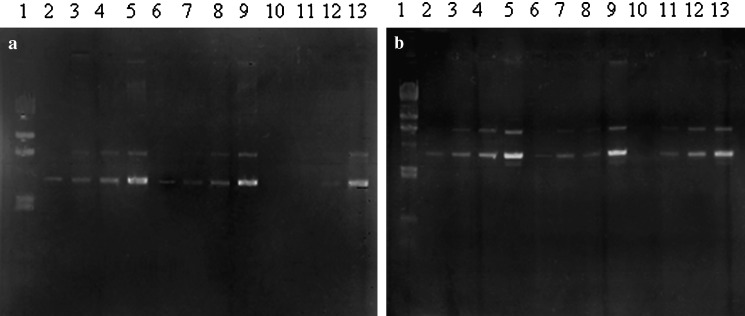
DNA-loaded pectinate nanoparticles were prepared by an ionic gelation procedure. pDNA at various amounts (0.01, 0.05, 0.1 or 0.5 mg) was incorporated into the pectinate submicron particles coded as F1 and F2. Aqueous solution of pectin containing pDNA was dropped into CaCl2 or MgCl2 solutions and pectinate nanoparticles were formed instantaneously by ionotropic gelation. With agarose gel electrophoresis, the ability of the pectinate particle to incorporate DNA was investigated. As seen in Fig. 2a, no unbound DNA was seen in lanes 10 or 11, confirming the results obtained from incorporation efficiency. Ca-pectinate nanoparticles (F1) could entrap up to 0.05 mg of DNA when the weight ratio of pectin:CaCl2:DNA was 0.2:1:0.05. However, Mg-pectinate (F2) could entrap only 0.01 mg of DNA when the weight ratio of pectin:MgCl2:DNA was 1:100:0.01 (Fig. 2b, lane 10). The particle size of pDNA-loaded Ca-pectinate particles (pectin/CaCl2/DNA = 0.2:1:0.05) was 944 ± 32 nm, whereas Mg-pectinate particles’s particle size (pectin/MgCl2/DNA = 1:100:0.01) was 650 ± 44 nm. Both formulations showed slightly negative charges, about −5 mV. The morphology of the particles was observed by TEM (Fig. 3). Both formulations had a spherical shape; the dry particle size was around 150–200 nm as determined by TEM. The mechanism by which pDNA was associated into the polymeric network was probably due to hydrogen bonding between pDNA and the amidate group on the pectin chain. Intermolecular cross-links were formed between the divalent calcium or magnesium ions and the negatively charged carboxyl groups of pectin molecules to form the characteristic egg-box structure (23).
Fig. 2.
Agarose gel electrophoresis of a pDNA-loaded Ca-pectinate, b pDNA-loaded Mg-pectinate. Lane1 Marker; lanes 2–5 0.01, 0.05, 0.1, and 0.5 μg of pDNA, respectively; lanes 6–9 pectin/CaCl2 = 0.1:0.5 or pectin/MgCl2 = 0.5:50 with 0.01, 0.05, 0.1, and 0.5 μg of pDNA, respectively; lanes 10–13 pectin/CaCl2 = 0.2:1.0 or pectin/MgCl2 = 1:100 with 0.01, 0.05, 0.1, and 0.5 μg of pDNA, respectively
In Vitro Gene Expression
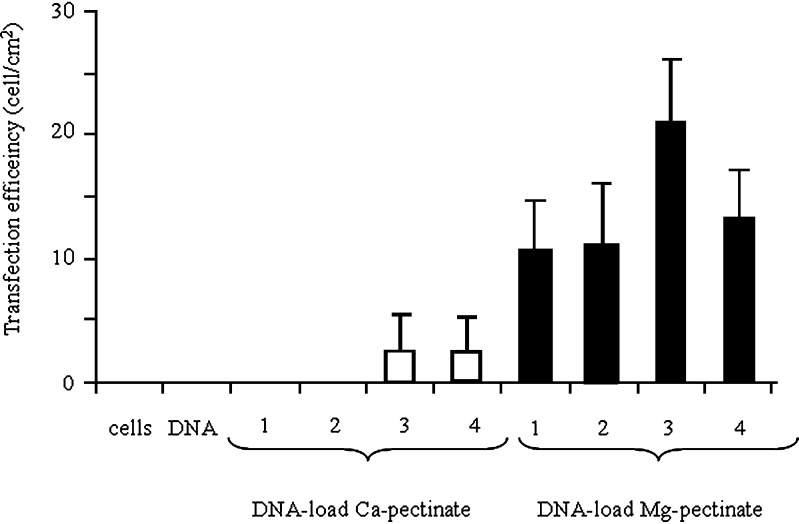
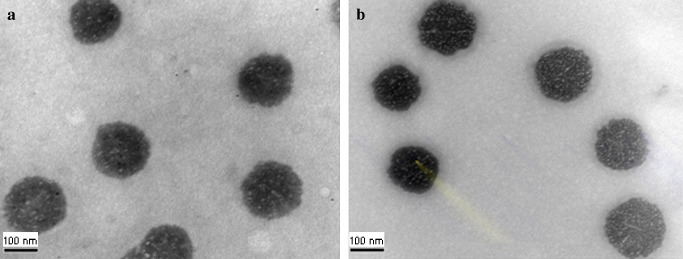
To determine the transfection ability of pectinate nanoparticles, a series of qualitative gene expression assays was performed in the Huh7 cell line using plasmid DNA-loaded pectinate nanoparticles prepared from a plasmid DNA-encoding EGFP. Fig. 4 shows fluorescence microscopy images of Huh7 cells 3 days after their exposure to free pDNA (negative control) or pDNA-loaded pectinate nanoparticles. For these experiments, cells were incubated with free pDNA or pDNA-loaded pectinate nanoparticles for 24 h in serum-free medium, followed by 48 h incubation in fresh serum-containing medium. These results demonstrated qualitatively that pectinate nanoparticles were capable of mediating the transfection of cells. The extent of transfection depended significantly upon the amount of pectinate nanoparticles. In this experiment, the ratio of pectin to DNA was varied at 1:1, 5:1, 10:1 and 20:1; the amount of DNA-loaded pectinate particles used to transfect cells was set at 1 μg/well. As shown in Fig. 5, both Ca-pectinate and Mg-pectinate nanoparticles gave relatively low levels of EGFP expression. The expression level of EGFP significantly increased with increasing amounts of pectinate particles. Mg-pectinate nanoparticles showed higher transfection efficiency than Ca-pectinate. It has been reported that the plasmid DNA encapsulated in either calcium phosphate or magnesium phosphate nanoparticles could be successfully used as a non-viral vector (28–29). The higher transfection efficiency of Mg-pectinate nanoparticles might be due to lower complexation reaction between magnesium ion and DNA including other biopolymers present in the system. However, this needs further investigation. Furthermore, the low transfection efficiency of Ca-pectinate nanoparticles might be due to the larger particle size of Ca-pectinate and the negative charge of those particles. Therefore, in future experiments, a cationic polymer such as polyethyleneimine (PEI) (30), poly-L-lysine (PLL) (21), or chitosan might be added to enhance the positive charge and the DNA loading efficiency, which might improve the DNA entrapment and transfection efficiency.
Fig. 4.
Fluorescence image observations for Huh7 cells’ expression of green fluorescence protein (GFP) after transfection of a free pDNA; b pDNA-loaded Ca-pectinate; c pDNA-loaded Mg-pectinate for 3 days. Magnification: ×10
Fig. 5.
Transfection efficiency of (open square) pDNA-loaded Ca-pectinate (pectin/CaCl2 = 0.2:1); (filled square) pDNA-loaded Mg-pectinate (pectin/MgCl2 = 0.01:1) in Huh7 cells. Each well has 1 μg of pDNA and pectin/pDNA (1 = 1:1; 2 = 5:1; 3 = 10:1; 4 = 20:1). Each value represents the mean±SD of three wells
Cytotoxicity of DNA-loaded Pectinate Nanoparticles
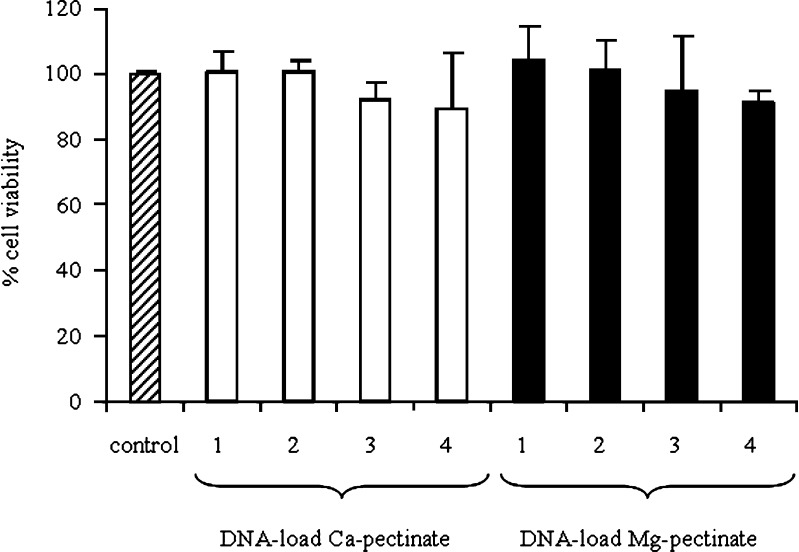
A cytotoxicity assay was performed in order to evaluate the potential of pectinate nanoparticles as vectors for safe gene delivery. Therefore, the cytotoxicity of the DNA-loaded pectinate nanoparticles at various concentrations was examined in Huh7 cells. Cells without pectinate nanoparticles treatment were considered to be a control, with a cell viability of 100%. Fig. 6 shows the effects of various concentrations of DNA-loaded pectinate nanoparticles on cell viability. The results showed that the cell viability of DNA-loaded pectinate nanoparticles formulated with Ca-pectinate and Mg-pectinate at various concentrations was not significantly different from the untreated cells (P > 0.05). Average cell viability was over 90%.
Fig. 6.
Cytotoxicity of (open square) pDNA-loaded Ca-pectinate (pectin:CaCl2 = 0.2:1); (filled square) pDNA-loaded Mg-pectinate (pectin/MgCl2 = 0.01:1) in Huh7 cells. Each well has 0.2 μg of pDNA and pectin/pDNA (1 = 1:1; 2 = 5:1; 3 = 10:1; 4 = 20:1). Each value represents the mean±SD of six wells
CONCLUSIONS
The concentration of both pectin and divalent cations (Ca2+, Mg2+ and Mn2+) affected the particle size and shape of pectinate micro/nanoparticles. The DNA-loaded Ca-pectinate and Mg-pectinate nanoparticles had the ability to transfect Huh7 cells with low cytotoxicity. This study suggests these pectinate nanoparticles have a potential use as safe gene delivery vectors.
ACKNOWLEDGMENTS
This work was supported by the Silpakorn University Research and Development Institute through the Pectin Research Program and the Office of the Commission for Higher Education, Ministry of Education, Thailand.
References
- 1.Zhang B., Xu Y. M., Wang B., Qiao W. H., Liu D. L., Li Z. S. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J. Control Release. 2004;100:165–180. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 2.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J. Control Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Rolland A. Gene medicines: the end of the beginning? Adv Drug Deliv Rev. 2005;57:669–673. doi: 10.1016/j.addr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Budker V., Gurevich V., Hagstrom J. E., Bortzov F., Wolff J. A. PH-sensitive, cationic liposomes: a new synthetic virus-like vector. Nature Biotech. 1996;14:760–764. doi: 10.1038/nbt0696-760. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Mounkes L. C., Liggitt H. D., Brown C. S., Solodin I., Heath T. D., Debs R. J. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nature Biotech. 1997;15:167–173. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- 6.Ruozi B., Forni F., Battini R., Vandelli M. A. Cationic liposomes for gene transfection. J. Drug Target. 2003;11:407–414. doi: 10.1080/10611860310001655600. [DOI] [PubMed] [Google Scholar]
- 7.Godbey W. T., Wu K. K., Mikos A. G. Poly(ethylenimine) and its role in gene delivery. J. Control Release. 1999;60:149–160. doi: 10.1016/S0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 8.Lungwitz U., Breunig M., Blunk T., Go-pferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Tang G. P., Guo H. Y., Alexis F., Wang X., Zeng S., Lim T. M., Ding J., Yang Y. Y., Wang S. Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. J Gene Med. 2006;8:736–744. doi: 10.1002/jgm.874. [DOI] [PubMed] [Google Scholar]
- 10.Pannier A. K., Shea L. D. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Csaba N., Sanchez A., Alonso M. J. PLGA:poloxamer and PLGA:poloxamine blend nanostructures as carriers for nasal gene delivery. J. Control Release. 2006;113:164–172. doi: 10.1016/j.jconrel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Garnett M. C. Gene-delivery systems using cationic polymers. Crit. Rev. Ther. Drug Carrier Syst. 1999;16:147–207. [PubMed] [Google Scholar]
- 13.C. Rolin. Pectin. In: R. L. Whistler, J. N. Bemiller (eds.), Industrial Gums: Polysaccharides and their Derivatives. New York: Academic; 1993:257–293.
- 14.Pillay V., Fassihi R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract. II. Physicochemical characterization of calcium-alginate, calcium-pectinate and calcium-alginate-pectinate pellets. J. Control Release. 1999;59:243–256. doi: 10.1016/S0168-3659(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 15.Sriamornsak P. Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur. J. Pharm. Sci. 1999;8:221–227. doi: 10.1016/S0928-0987(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 16.Kim T. H., Park Y. H., Kim K. J., Cho C. S. Release of albumin from chitosan-coated pectin beads in vitro. Int. J. Pharm. 2003;250:371–383. doi: 10.1016/S0378-5173(02)00553-7. [DOI] [PubMed] [Google Scholar]
- 17.Atyabi F., Inanloo K., Dinarvand R. Bovine serum albumin-loaded pectinate beads as colonic peptide delivery system: preparation and in vitro characterization. Drug Deliv. 2005;12:367–375. doi: 10.1080/10717540590968666. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois S., Laham A., Besnard M., Andremont A., Fattal E. In vitro and in vivo evaluation of pectin beads for the colon delivery of beta-lactamases. J. Drug Target. 2005;13:277–284. doi: 10.1080/10611860500206583. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois S., Gernet M., Pradeau D., Andremont A., Fattal E. Evaluation of critical formulation parameters influencing the bioactivity of beta-lactamases entrapped in pectin beads. Int. J. Pharm. 2006;324:2–9. doi: 10.1016/j.ijpharm.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K., Lim L. Y. Insulin-loaded calcium pectinate nanoparticles: effects of pectin molecular weight and formulation pH. Drug Dev. Ind. Pharm. 2004;30:359–367. doi: 10.1081/DDC-120030930. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez Ferreiro M., Tillman L., Hardee G., Bodmeier R. Characterization of alginate/poly-L-lysine particles as antisense oligonucleotide carriers. Int. J. Pharm. 2002;239:47–59. doi: 10.1016/S0378-5173(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 22.Smith M. D., Barbenel J. C., Courtney J. M., Grant M. H. Novel quantitative methods for the determination of biomaterial cytotoxicity. Int. J. Artif. Organs. 1992;15:191–194. [PubMed] [Google Scholar]
- 23.Braccini I., Perez S. Molecular basis of Ca (2+)-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules. 2001;2:1089–1096. doi: 10.1021/bm010008g. [DOI] [PubMed] [Google Scholar]
- 24.Norgard M. V., Keem K., Monahan J. J. Factor affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978;3:279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- 25.Popowski J., Bednarska M. Mn2+ dependent transformation of Bacillus subtilis. Acta Microbiol. Pol. 1985;34:217–225. [PubMed] [Google Scholar]
- 26.Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. U S A. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang R., Reusch R. N. Genetic competence in Escherichia coli requires poly-β-hydroxybutyrate/calcium polyphosphate membrane complexes and certain divalent cations. J Bacteriol. 1995;177:486–490. doi: 10.1128/jb.177.2.486-490.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhakta G., Mitra S., Maitra A. DNA encapsulated magnesium and manganous phosphate nanoparticles: potential non-viral vectors for gene delivery. Biomaterials. 2005;26:2157–2163. doi: 10.1016/j.biomaterials.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 29.Bisht S., Bhakta G., Mitra S., Maitra A. N. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viralvector for gene delivery. Int. J. Pharm. 2005;288:157–168. doi: 10.1016/j.ijpharm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Patnaik S., Aggarwal A., Nimesh S., Goel A., Ganguli M., Saini N., Singh Y., Gupta K. C. PEI-alginate nanocomposites as efficient in vitro gene transfection agents. J. Control Release. 2006;114:398–409. doi: 10.1016/j.jconrel.2006.06.025. [DOI] [PubMed] [Google Scholar]