Table V.
Thermodynamic Functions of Solubility and Solvation Processes of 2-, 3-, and 4-Acetaminophens in Solvents Studied
| Solvent |
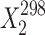 a (mol frac)
a (mol frac) |
 b (kJ mol−1)
b (kJ mol−1) |
 c (kJ mol−1)
c (kJ mol−1) |
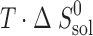 (kJ mol−1) (kJ mol−1) |
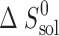 (J K−1 mol−1) (J K−1 mol−1) |
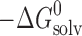 d (kJ mol−1)
d (kJ mol−1) |
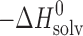 e (kJ mol−1)
e (kJ mol−1) |
 (kJ mol−1) (kJ mol−1) |
 (J K−1 mol−1) (J K−1 mol−1) |
ζHsolv (%) | ζTSsolv (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Acetaminophen | |||||||||||
| n-Hexane | 4.98 × 10−6 | 30.3 | 36.6 ± 0.9 | 6.3 | 21 ± 1 | 22.5 | 85.2 | 62.7 | 210 | 57.6 | 42.4 |
| Water | 1.34 × 10−4 | 22.1 | 20.7 ± 0.7 | −1.4 | −4.7 ± 1 | 30.7 | 101.1 | 70.4 | 236 | 59.0 | 41.0 |
| n-Octanol | 5.63 × 10−3 | 12.8 | 24.6 ± 0.7 | 11.8 | 40 ± 1 | 40.0 | 97.2 | 57.2 | 192 | 63.0 | 37.0 |
| 3-Acetaminophen | |||||||||||
| n-Hexane | 5.13 × 10−6 | 30.2 | 53.7 ± 1.7 | 23.5 | 79 ± 1 | 26.2 | 56.3 | 30.1 | 101 | 65.2 | 34.8 |
| Water | 1.58 × 10−3 | 16.0 | 35.6 ± 0.9 | 19.6 | 66 ± 1 | 40.4 | 75.4 | 35.0 | 117 | 68.3 | 31.7 |
| n-Octanol | 3.88 × 10−2 | 8.1 | 18.2 ± 0.3 | 10.1 | 34 ± 1 | 48.3 | 92.8 | 44.5 | 149 | 67.6 | 32.4 |
| 4-Acetaminophene | |||||||||||
| n-Hexane | 4.12 × 10−6 | 30.7 | 41.9 ± 0.7 | 11.2 | 37.6 | 29.3 | 76.0 | 46.7 | 157 | 61.9 | 38.1 |
| Water | 1.72 × 10−3 | 15.8 | 21.8 ± 0.1 | 6.0 | 20.1 | 44.2 | 96.1 | 51.9 | 174 | 64.9 | 35.1 |
| n-Octanol | 2.47 × 10−2 | 6.0f | 11.7 ± 0.2 | 5.7 | 19.1 | 54.0 | 106.2 | 52.2 | 175 | 65.5 | 34.5 |
aThe experimental error within 2%
bCalculated from the temperature dependence of solubility data
c

d
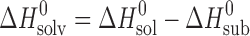
ePerlovich et al. (4)
fTaking into account activity coefficient
