Abstract
The purpose of present study was to evaluate commercial preparations of carbamazepine tablets with respect to drug release through a defined sequence of experiments using Minitab software. The compliance of products with respect to United States Pharmacopeia (USP) dissolution test and comparison of the products with respect to drug release in different dissolution conditions is reported in the present paper. The different dissolution conditions studied include dissolution medium (1% SLS in purified water, 0.1 N HCl), volume (900 and 1,000 ml), rpm (50 rpm, 75 rpm). Studies indicated that all six products complied with USP dissolution criteria. However, the extent of influence of dissolution conditions on drug release was varied among the products. Distinct dissolution profiles were observed and there was no correlation with disintegration time in certain products. The in vitro dissolution experimentation helped in identifying the discriminatory dissolution conditions and also the formulations that were unaffected with change of dissolution variables. In summary, commercial preparations of carbamazepine vary widely in their dissolution behavior in multi dissolution run experimentation. Identifying this behavior of the products was essential as an in vitro tool for screening a good and a bad formulation.
Key words: carbamazepine, dissolution, drug release, sodium lauryl sulfate
INTRODUCTION
Carbamazepine (CBZ) is a widely used anti epileptic drug. It is BCS class II drug practically insoluble in water (∼113 μg/ml) that has dissolution dependent oral bioavailability (1). The gastrointestinal absorption is characterized as slow, erratic and possibly incomplete (2).
In addition the rate of absorption of CBZ can differ markedly with different pharmaceutical formulations (3,4). Loss of seizure control and occurrence of side effects in many cases have been reported when one CBZ immediate release product is exchanged for another (5). In another study on the pharmaceutical quality of CBZ immediate release tablets, it is reported that differences were observed in dissolution rate even within a single brand (6). Carbamazepine is highly sensitive to moisture in tablets, resulting in a change in the dissolution rate in vitro and in vivo (7–10).
Jung et al. (11), have carried out in vitro and in vivo studies for carbamazepine commercial formulations and found that United States Pharmacopeia (USP) XX VI in vitro dissolution method cannot be used to accurately predict the bioavailability of a carbamazepine formulation and suggested for additional work in order to obtain good in vitro and in vivo correlation.
Literature has mentioned usage of more than two dissolution media (1% sodium lauryl sulfate (SLS), 0.1 N Hydrochloric acid (HCl) and water) for dissolution studies of carbamazepine tablets (12–16). In another report it was mentioned that 1% SLS and 0.1 N HCl were preferred on the basis of IVIVC studies (13). Dissolution of poorly water soluble drugs in presence of surfactant is reported in the literature (17). It is difficult to make a good estimate with a single dissolution study in view of reported factors influencing the dissolution of carbamazepine tablets. The above-mentioned reports show the importance of in vitro dissolution as a tool for evaluation of the performance and consistency in quality of commercial preparations of carbamazepine. Therefore, the present study of generation and evaluation of dissolution data for carbamazepine products is of significance.
MATERIALS AND METHODS
Materials
Carbamazepine was a gift from Sun Pharmaceuticals Ltd. Sodium lauryl sulfate (HiMedia, India), hydrochloric acid (Merck, India) and other chemicals of analytical reagent grade were purchased. Six brands of 200 mg carbamazepine tablets were obtained through a local pharmacy store and are designated as product A, B, C, D, E, and F. The brands are Neurocarb-200, Lot no. NCB 4002 (Product A), Mazetol-200, Lot no. 5A 811(Product B), Zen-200, Lot no. EO480 (Product C), Tegrital-200, Lot no. 53033V (Product D), Carbatol-200, Lot no. B1835001 (Product E) and Mezapin, Lot no. M/243 (Product F).
Methods
Determination of CBZ Solubility
An excess amount (40 mg) of carbamazepine was added to a screw-capped vial containing 10 ml of a dissolution medium and was shaken on a rotary shaker for 24 h at 25°C. At the end of 24 h the samples were filtered through 0.45 μm membrane filter (Millipore) and diluted before UV–Vis spectrophotometer (Elico SL 159, Elico India Ltd, India) analysis for the drug content.
Characterization
Selected brands of carbamazepine 200 mg tablets of different manufacturers were characterized with respect to tablet shape, average weight, disintegration time, and moisture content. The products were subjected to disintegration at 37 ± 0.5°C on a disintegration tester (Electrolab, India). Moisture content of tablets was determined on crushed samples at 105°C for 10 min using IR moisture balance (Sartorius MA 100 Sartorius, Germany).
Dissolution Study
The United States Pharmacopeia (USP) dissolution apparatus II (Paddle method) was utilized to conduct all the dissolution tests (18). Dissolution runs were carried out on six individual units using dissolution tester (Disso 2000; Labindia, India) with vessels stirred at the respective rpm of the run, while temperature of the dissolution media was kept at 37 ± 0.5°C throughout the dissolution study. Samples of 5 ml were withdrawn at 15, 30, 45 and 60 min and were replaced with equal volume of fresh media. Aqueous samples were passed through 0.45 μm membrane filter (Millipore) and diluted before absorbance was measured at 288 nm using UV–Vis Spectrophotometer (Elico SL 159, Elico India Ltd, India).
Dissolution runs were carried for six carbamazepine products in a random order generated with Minitab software. Eight different dissolution runs were carried out for each product with three dissolution variables studied each at two levels. Three dissolution variables namely dissolution volume (900 ml and 1,000 ml), rpm (50 and 75) and medium (1% SLS in water and 0.1 N HCl) were studied. The two levels selected for each dissolution variable was with in the normal range of dissolution studies and details of dissolution runs are shown in Table I. During each dissolution run the physical observations of tablets such as breaking pattern were recorded for the purpose of correlation with drug release.
Table I.
Dissolution Runs of Carbamazepine Formulations
| Dissolution Run | Dissolution Medium | RPM | Volume (ml) |
|---|---|---|---|
| 1 | 1% SLS | 50 | 900 |
| 2 | 1% SLS | 50 | 1,000 |
| 3 | 1% SLS | 75 | 900 |
| 4 | 1% SLS | 75 | 1,000 |
| 5 | 0.1 N HCl | 50 | 900 |
| 6 | 0.1 N HCl | 50 | 1,000 |
| 7 | 0.1 N HCl | 75 | 900 |
| 8 | 0.1 N HCl | 75 | 1,000 |
Dissolution Data Analysis
The dissolution data generated for each product in different dissolution runs were processed with Minitab software (Minitab 14, Minitab, PA, USA) and plots were generated for interpretation of the dissolution data. Analysis of variance (ANOVA) was employed to determine extent of variability in dissolution profiles among the products and also variability of the product in different dissolution runs using Graphpad Instat software (Graphpad 4.03 version).
RESULTS AND DISCUSSION
Solubility Studies
The aqueous solubility of pure drug was found to be 3.412 ± 0.13 and 0.214 ± 0.05 mg/ml in purified water with 1% SLS and in 0.1 N HCl respectively. The values are in accordance with the reported values (15). In 0.1 N HCl medium the solubility was significantly low compared to that of surfactant medium. However, near to a sink condition could be attained in 900 ml of 0.1 N HCl medium for one CBZ tablet with 200 mg dose unit.
Product Characteristics
All carbamazepine products were characterized before dissolution experimentation. Tablet shape, average weight, disintegration time, moisture content and pack details were recorded for all the brands and are shown in Table II. All products were uncoated tablets and white in color. Four products were round shaped and two products are caplet shaped tablets. Caplet shaped tablets is strip packed where as round shaped tablets are in blister pack. Average weight and disintegration of products was in the range of 232.7 to 321.1 mg and 0.4–4.3 min respectively. Product C has lowest weight and product A has highest tablet weight while product D has least disintegration time and product E has highest disintegration time. Moisture content of the products was in the range of 1.51% to 3.95% w/w. The physical characterization data show a certain degree of variability among the brands.
Table II.
Product Characteristics of Carbamazepine 200 mg Tablets
| Product | Brand Name | Pack | Shape | Average Weight (mg) ± SD | DT (min) ± SD | Moisture content (%) ± SD |
|---|---|---|---|---|---|---|
| A | Neurocarb-200 | Strip | Caplet | 321.1 ± 2.1 | 3.5 ± 1.4 | 2.59 ± 1.1 |
| B | Mazetol-200 | Blister | Round | 271.6 ± 1.5 | 2.3 ± 2.1 | 3.95 ± 1.8 |
| C | Zen-200 | Blister | Round | 232.7 ± 3.4 | 3.7 ± 2.5 | 2.41 ± 0.8 |
| D | Tegrital-200 | Strip | Caplet | 278.4 ± 1.4 | 0.4 ± 0.7 | 1.51 ± 1.4 |
| E | Carbatol-200 | Blister | Round | 237.5 ± 2.8 | 4.3 ± 2.3 | 2.22 ± 1.3 |
| F | Mezapin | Blister | Round | 285.6 ± 4.2 | 2.8 ± 1.8 | 1.89 ± 0.5 |
DT Disintegration time
Dissolution Study
In the present study, carbamazepine tablets 200 mg of different brands available in the market were evaluated with respect to drug release. Dissolution profiles were generated in different dissolution conditions. These elaborative studies are required for carbamazepine, a BCS class II drug with wide differences reported in the literature with respect to clinical effects by switch over from one brand to brand. Reproducible and sufficient release of these compounds can only be guaranteed by careful formulation and high quality manufacturing procedures. Eight dissolution runs (three variables at two levels) were carried out through Minitab design in a random order with dissolution conditions of each run shown in Table I.
In the first instance the dissolution data of run 3 was reviewed for compliance of USP dissolution specifications. USP XXVI criteria for dissolution of carbamazepine tablets are; drug dissolved (%) should be between 45–75% in 15 min and not less than 80% in 60 min. All six carbamazepine products have passed the USP dissolution specifications of not less than 80% drug release in 60 min. Among the products variability was higher in tablets of product B. This variability might be due to poor formulation of the product.
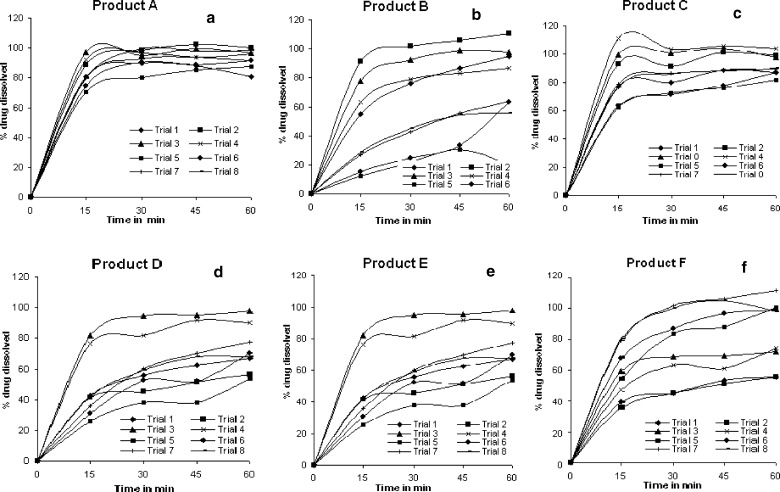
Drug dissolved (%) at 60 min as part of specifications of carbamazepine tablets in USP was determined for all the dissolution runs and based on the release values they were assigned pass or fail and tabulated in Table III. From the Table III, it was evident that products A, C and E complied with USP dissolution specifications in all the dissolution runs while product D complies only in three and four runs. An interesting observation was found in case of products B and F. Product B passes USP dissolution test in all dissolution runs with 1% SLS as dissolution medium while product F passes with 0.1 N HCl as dissolution medium. In addition to evaluation of products compliance with USP, variability among the products was also determined and for this purpose comparative dissolution profiles were plotted in Fig. 1. Variability was least in dissolution profiles of product A while it was highest in product B. In support of this standard deviation was determined on mean drug release of each product in eight dissolution runs and the values are shown in Table IV. Similar observations were seen with standard deviation values indicating minimum variability for profiles of product A and maximum in case of product B in comparison to other products.
Table III.
USP Compliance of Six Brands of Carbamazepine Tablets 200 mg
| Run | Conditions | Products | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| 1 | 1% SLS, 900 ml, 50 rpm | Pass | Pass | Pass | Fail | Pass | Fail |
| 2 | 1% SLS, 1,000 ml, 50 rpm | Pass | Pass | Pass | Fail | Pass | Fail |
| 3 | 1% SLS, 900 ml, 75 rpm | Pass | Pass | Pass | Pass | Pass | Pass |
| 4 | 1% SLS, 1,000 ml, 75 rpm | Pass | Pass | Pass | Pass | Pass | Pass |
| 5 | 0.1 N HCl, 900 ml, 50 rpm | Pass | Fail | Pass | Fail | Pass | Pass |
| 6 | 0.1 N HCl, 1,000 ml, 50 rpm | Pass | Pass | Pass | Fail | Pass | Pass |
| 7 | 0.1 N HCl, 900 ml, 75 rpm | Pass | Fail | Pass | Fail | Pass | Pass |
| 8 | 0.1 N HCl, 1,000 ml, 75 rpm | Pass | Fail | Pass | Fail | Pass | Pass |
Fig. 1.
Comparative dissolution profiles of carbamazepine products in different dissolution runs a product A, b product B, c product C, d product D, e product E, and f product F
Table IV.
Standard Deviationa values of carbamazepine brands
| Time (min) | Product A | Product B | Product C | Product D | Product E | Product F |
|---|---|---|---|---|---|---|
| 15 | 9.00 | 29.72 | 17.04 | 20.67 | 17.97 | 16.97 |
| 30 | 6.23 | 30.87 | 11.81 | 18.67 | 20.91 | 22.45 |
| 45 | 6.04 | 29.15 | 11.36 | 19.76 | 21.48 | 22.83 |
| 60 | 6.32 | 29.06 | 7.56 | 15.29 | 17.33 | 21.77 |
aStandard deviation was determined on % drug dissolved in eight runs
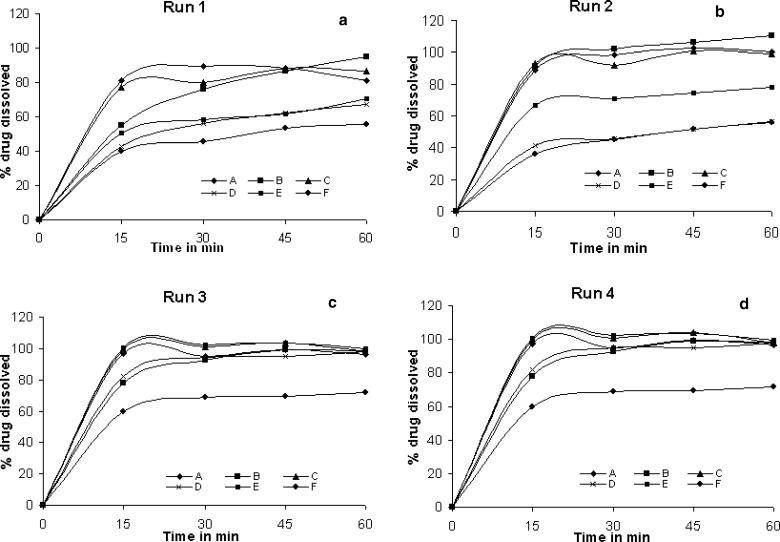
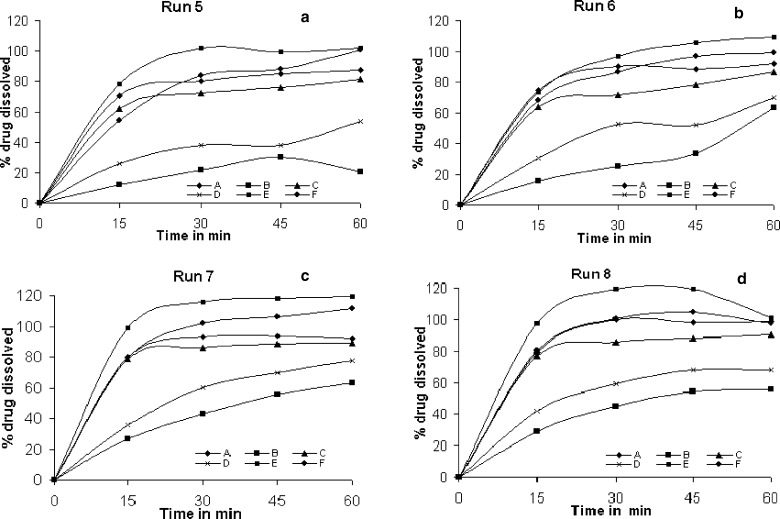
In another attempt to estimate the extent of discrimination among the products in a particular dissolution run, standard deviation was calculated for the % drug release of the six products in a particular dissolution run and the values are shown in Table V and comparative profiles are shown in Figs. 2 and 3. Standard deviation was least in run 3 as seen from the similar release profiles while maximum variation in drug release among the products was seen in dissolution run 5 (0.1 N HCl, 900 ml, 50 rpm). The difference in dissolution profiles among the products in each dissolution run was found to be statistically significant (p < 0.0001). Similarly difference in profiles of individual product in different dissolution runs was found to be statistically significant (p < 0.0001). Upon changing the dissolution condition it is obvious to influence the drug release especially in case of class II drugs. But in case of CBZ brands the extent of influence was enormous among the brands. Difference in profiles can probably be explained by a combination of variability in particle size of the active ingredient, the use of different excipients, polymorphic transitions and differences in the manufacturing process. The drug is reported to exist in different polymorphic forms and polymorphic transformation takes place due to moisture content in the tablets (7). In case of carbamazepine brands the wide range of profiles among the brands could be due to either one or combination of these factors. In the present study the polymorphic form of drug in the brands was not determined. However, if polymorphic form and coexistence of polymorphs exist and differ among the brands the influence should have been visible in the dissolution profiles of run 3 (Dissolution as per USP). Hence the difference in release profiles among the brands in other dissolution runs could not be ascertained to polymorphic differences.
Table V.
Comparison of Standard Deviationa Values of Dissolution Runs
| Time (min) | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 | Run 8 |
|---|---|---|---|---|---|---|---|---|
| 15 | 17.52 | 25.84 | 16.13 | 22.57 | 26.08 | 25.03 | 28.47 | 26.25 |
| 30 | 16.87 | 25.78 | 12.23 | 14.42 | 30.33 | 27.41 | 27.03 | 27.99 |
| 45 | 15.96 | 25.61 | 13.09 | 14.86 | 28.44 | 27.71 | 23.14 | 25.80 |
| 60 | 14.25 | 23.52 | 10.68 | 11.04 | 31.63 | 17.39 | 22.66 | 18.90 |
aStandard deviation was determined on % drug dissolved of six carbamazepine brands
Fig. 2.
Comparative dissolution profiles of carbamazepine products A, B, C, D, E & F; a run 1, b run 2, c run 3, and d run 4
Fig. 3.
Comparative dissolution profiles of carbamazepine products A, B, C, D, E & F; a run 5, b run 6, c run 7, and d run 8
Dissolution behavior of tablets was observed visually during dissolution studies. In case of product A fine granules were seen upon disintegration in the dissolution vessel and dissolution medium was clear at the end of dissolution. The formulation might have major portion of soluble excipients and this might be one of the reason for having low variability in terms of drug release for product A compared to other products. Also as seen from the average weight data in Table II, the product A has highest tablet weight that might result in improved dissolution characteristics with minimum variability. The interpretation that could be drawn in product B is a different one. These tablets disintegrated faster when dissolution runs were carried out in 1% SLS media while the disintegration was very slow in 0.1 N HCl media and this was reflected on drug release in the respective media. There might be multiple reasons for the failure of the product however, it can be concluded because of poor formulation composition when compared to other products dissolution profiles.
The correlation between disintegration test and release profile is difficult to establish in certain carbamazepine products. Disintegration of product D tablets was least (0.4 min) among the commercial products of carbamazepine tablets studied. However, this was not reflected on mean drug release while in product E the time taken for tablets to disintegrate in the dissolution vessel was higher compared to other products. In spite of this pattern the dissolution rate of product E was higher than other products. The differences among the products surfaced out due to extensive dissolution studies. No correlation of disintegration time with in vitro dissolution was evident. Slow dissolution of drug from the granules and nature of granules contribute for having no correlation and this calls for reformulation in order to achieve uniform and better dissolution. The dissolution data obtained will be of help in screening and identifying a good and a bad formulation with respect to bio success.
The following are the observations that were seen with change in one of the dissolution parameter on drug release from the marketed preparations. The influence of change in dissolution volume from 900 to 1,000 ml and increase in rpm from 50 to 75 rpm has resulted in increased drug release from all the products. Since carbamazepine is a class II drug with low aqueous solubility, slight increase in dissolution volume might have provided better sink conditions while increase in rpm might have improved the hydrodynamics and thereby prevented heap formation at the bottom of dissolution vessel in certain products.
The influence of dissolution medium on drug release was different in a sense that drug dissolved (%) was higher in 1% SLS for some of the products while others shown higher release in 0.1 N HCl medium. From the solubility data, ideally all the products should show faster rate and complete dissolution in surfactant medium than that of 0.1 N HCl. It was found that at 900 ml and 50 rpm, the mean drug release was more in 1% SLS than that of 0.1 N HCl medium in all the products. Based on the dissolution data, 0.1 N HCl demonstrated sufficient discriminating power among the brands. Difference in profiles can be probably explained by a combination of factors. Solvang showed, for example, that use of different binders influences the dissolution behavior of phenobarbital from tablet formulations to a large extent (19). Through these dissolution findings, products A, C and E have a greater probability of in vivo success compared to B, D and F products.
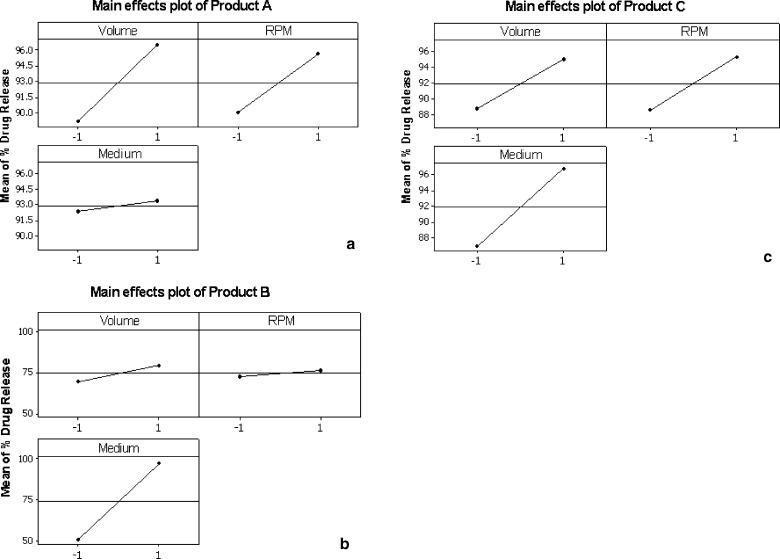
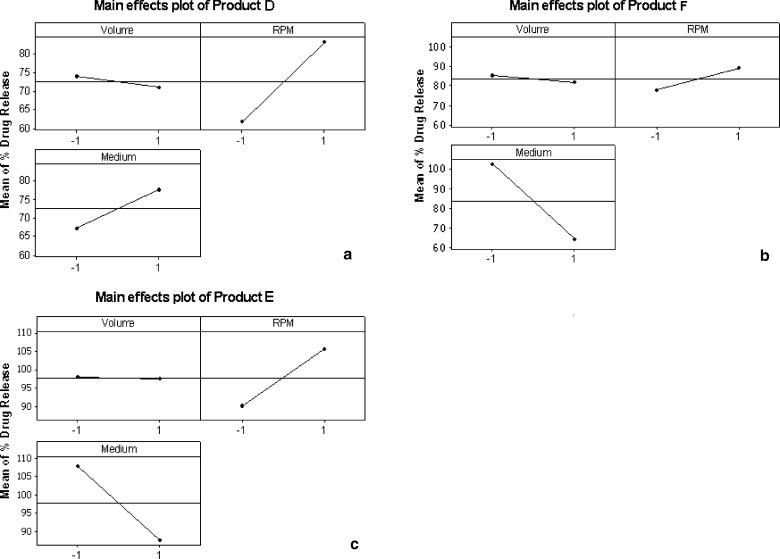
The influence of change in dissolution condition on drug release from the tablets is imminent; however the extent of influence on each product is of interest in the present study. This interpretation was done with the help of main effects plots generated through Minitab software as shown in Fig. 4 for products A, B, C and Fig. 5 for D, E, F respectively. The main effects plot consists of mean drug release (%) in 60 min on y-axis and the two levels of dissolution variables (volume, rpm and medium) denoted as −1 and +1 on x-axis. The drug release (%) shown on y-axis is mean of drug release obtained in all dissolution runs that were run at two levels of a particular variable. For instance, the mean of drug release of four dissolution runs with 900 ml medium denoted as “−1” and 1,000 ml medium denoted as “+1” is shown in a plot of mean drug release (%) on y-axis and volume on x-axis. The angle of the line joining the two levels in each plot indicates the extent of influence of each dissolution variable on drug release. The main effects plots helped in identifying and concluding that the effect of change in dissolution volume and rpm had different influence on drug release among the products.
Fig. 4.
Main effects plots of mean % drug release versus dissolution variables (volume, rpm, and medium) of carbamazepine products; a product A, b product B, and c product C
Fig. 5.
Main effects plots of mean % drug release versus dissolution variables (volume, rpm, and medium) of carbamazepine products; a product D, b product E, and c product F
The influence of dissolution medium has negligible effect on drug release (%) in product A while in case of product B it has significant influence as seen from the angle of the line joining the two levels in the plot (Fig. 4). Due to this difference in drug release in both media the standard deviation was highest for product B. In case of product C change in level of all the three variables has similar extent of influence on drug release (%). This can be seen from the Fig. 4 where the angle of the lines joining the two points was almost similar in all the three cases. In case of product D, E and F the level of dissolution volume has negligible influence on drug release (%). It can be seen in Fig. 5 plots for product E and F, that the line joining the two points in dissolution medium variable was from top to bottom indicating that the drug release (%) was decreased when SLS medium was replaced with 0.1 N HCl.
From the observations in the present study, it was evident that all brands comply with USP dissolution specification. However, on the basis of this method alone the accurate bioavailability of the products cannot be predicted, as it was not discriminatory. Hence the present additional dissolution data of different brands with respect to rate and extent of drug release is of importance. The suitability of these dissolution conditions for IVIVCs will require further validation through a bio study with slow, medium and fast releasing carbamazepine formulations.
The observed differences in dissolution results at different experimental conditions were not due to inherent variability of the technique but might be due to the nature of formulation. This could be observed from the dissolution profiles where in certain brands did not show any significant difference at different experimental conditions. The robustness of the formulations i.e., with respect to its dissolution could be judged from different dissolution runs carried out with Minitab software which was the other objective in addition to evaluation of USP compliance.
CONCLUSION
The present investigation demonstrates that the generation of exhaustive dissolution data on carbamazepine marketed preparations differentiated a good and a bad formulation. The dissolution behaviour varied among the products and these findings with further support of an in vivo study will be of significance.
Acknowledgments
The authors would like to thank Mr. B.C. Rajesha, Apotex India Ltd, Bangalore for his support in experimental design and Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance.
References
- 1.Clarke A. Isolation and identification of drugs. 2nd ed. London, UK: The Pharmaceutical Press; 1986. [Google Scholar]
- 2.Riad L. E., Chan K. K., Wagner W. E. Simultaneous first and zero order absorption of Carbamazepine tablets in humans. J. Pharm. Sci. 1986;75:897–900. doi: 10.1002/jps.2600750916. [DOI] [PubMed] [Google Scholar]
- 3.Aldenkamp A. P., Rentmeester T., Hulsman J., et al. Pharmacokinetics and cognitive effects of Carbamazepine formulations with different dissolution rates. Eur. J. Clin. Pharm. 1998;54:184–192. doi: 10.1007/s002280050443. [DOI] [PubMed] [Google Scholar]
- 4.J. E. F. Reynolds, and A. B. Prasad. Martindale: The Extra Pharmacopoeia. 28th Edn., The Pharmaceutical Press, London, (1982).
- 5.Olling M., Mensinga T. T., Barends D. M., Groen C., Lake O. A., Meulenbelt J. Bioavailability of Carbamazepine from four different products and the occurrence of side effects. Biopharm. Drug Dispos. 1999;20:19–28. doi: 10.1002/(SICI)1099-081X(199901)20:1<19::AID-BDD152>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Davidson A. G., Breslin F., Lancashire H. J., Mossop W. J., Alexander R. G. A multinational survey of the quality of Carbamazepine tablets, Medicines testing laboratory. UK: Edinburgh; 1993. [Google Scholar]
- 7.Bell W. L., Crawford I. L., Shiu G. K. Reduced bioavailability of moisture-exposed Carbamazepine resulting in status epilepticus. Epilepsia. 1993;34:1102–1104. doi: 10.1111/j.1528-1157.1993.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer M. C., Straughn A. B., Jarvi E. J., Wood G. C., Pelsor F. R., Shah V. P. The bioinequivalence of Carbamazepine tablets with a history of clinical failures. Pharm. Res. 1992;9:1612–1616. doi: 10.1023/A:1015872626887. [DOI] [PubMed] [Google Scholar]
- 9.Wang J. T., Shiu G. K., Ong-Chen T., Vishwanathan C. T., Skelly J. P. Effects of humidity and temperature on in vitro dissolution of Carbamazepine tablets. J Pharm Sci. 1993;83:1002–1005. doi: 10.1002/jps.2600821006. [DOI] [PubMed] [Google Scholar]
- 10.Zakkinios P. N., Li W. I., Drennen J. K., Lodder R. A. Spectrophotometric prediction of the dissolution rate of Carbamazepine tablets. Pharm. Res. 1991;8:974–978. doi: 10.1023/A:1015840604423. [DOI] [PubMed] [Google Scholar]
- 11.Jung H., Milan R. C., Girard M. E., Leon F., Montayo M. A. Bioequivalence study of Carbamazepine tablets: in vitro/in vivo correlation. Int. J. Pharm. 1997;152:37–44. doi: 10.1016/S0378-5173(97)04910-7. [DOI] [Google Scholar]
- 12.Lake O. A., Olling M., Barends D. M. In vitro/in vivo correlation of dissolution data of Carbamazepine immediate release tablets with pharmacokinetic data obtained in healthy volunteers. Eur. J. Pharm. Biopharm. 1999;48:13–19. doi: 10.1016/S0939-6411(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 13.Meyer M. C., Straughn A. B., Mahatre R. M., Shah V. P., Williams R. L. The relative bioavailability and in vivo–in vitro correlation for four marketed Carbamazepine tablets. Pharm. Res. 1998;15:1787–1791. doi: 10.1023/A:1011929300613. [DOI] [PubMed] [Google Scholar]
- 14.El-Zein H., Riad L., El-Berry A. A. Enhancement of Carbamazepine dissolution: in vitro and in vivo correlation. Int. J. Pharm. 1998;168:209–220. doi: 10.1016/S0378-5173(98)00093-3. [DOI] [Google Scholar]
- 15.Lee H, Park S. A., Sah H. Surfactant effects upon dissolution patterns of Carbamazepine immediate release tablets. Arch. Pharm. Res. 2005;28:120–126. doi: 10.1007/BF02975147. [DOI] [PubMed] [Google Scholar]
- 16.Kaneniwa N., Umezawaa O., Watari N., Kawatami K., Asami H. Bioavailability and dissolution test of commercial Carbamazepine tablets. Yakugaku Zasshi. 1984;104:83–90. doi: 10.1248/yakushi1947.104.1_83. [DOI] [PubMed] [Google Scholar]
- 17.Shah V. P., Konecny J. J., Everett R. L., McCullogh B., Noorizadeh A. C., Skelly J. P. In vitro dissolution profile of water insoluble drug dosage forms in the presence of surfactants. Pharm. Res. 1989;6:612–618. doi: 10.1023/A:1015909716312. [DOI] [PubMed] [Google Scholar]
- 18.United States Pharmacopoeia, XXVI, United States, United States Pharmacopoeial Convention, Inc. 324–325 (2003).
- 19.Solvang S., Finholt P. Effect of tablet processing and formulation factors on dissolution rate of the active ingredient in human gastric juice. J. Pharm. Sci. 2006;59:49–52. doi: 10.1002/jps.2600590106. [DOI] [PubMed] [Google Scholar]