Abstract
Buccal bioadhesive films, releasing topical drugs in the oral cavity at a slow and predetermined rate, provide distinct advantages over traditional dosage forms. The aim of present study was to prepare and evaluate buccal bioadhesive films of clotrimazole for oral candidiasis. The film was designed to release the drug at a concentration above the minimum inhibitory concentration for a prolonged period of time so as to reduce the frequency of administration of the available conventional dosage forms. The different proportions of sodium carboxymethylcellulose and carbopol 974P (CP 974P) were used for the preparation of films. Carbopol was used to incorporate the desired bioadhesiveness in the films. The films were prepared by solvent casting method and evaluated for bioadhesion, in vitro drug release and effectiveness against Candida albicans. In vitro drug release from the film was determined using a modified Franz diffusion cell while bioadhesiveness was evaluated with a modified two-arm balance using rabbit intestinal mucosa as a model tissue. Films containing 5% CP 974P of the total polymer were found to be the best with moderate swelling along with favorable bioadhesion force, residence time and in vitro drug release. The microbiological studies revealed that drug released from the film could inhibit the growth of C. albicans for 6 h. The drug release mechanism was found to follow non-Fickian diffusion.
Key words: buccal bioadhesive films, carbopol 974P, clotrimazole, SCMC
INTRODUCTION
Candidiasis in the oral cavity is an opportunistic infectious condition caused by a ubiquitous, saprophytic fungus of the genus Candida, the most common of which is Candida albicans. C. albicans is a resident commensal fungus of the normal oral flora. It can infect when predisposing factors such as antibiotic therapy, corticosteroid therapy, xerostomia (dry mouth), diabetes mellitus, chemo/radiation therapy, and immunosuppression are present. Recently the advent of the human immunodeficiency virus infection has resulted in a resurgence of oral Candida infections. General debilitation, poor oral or dental hygiene, and ill-fitted dentures are some of the other predisposing factors responsible for the cause of oral candidiasis. Fungal opportunistic infections, including oral candidiasis, are a major cause of morbidity and mortality in cancer patients (1–4). Chronic antimycotic therapy in high doses is undesirable for treatment of oral infections due to potential side effects. Therefore, to minimize these adverse effects and the ominous risk of drug resistance, topical therapy should be considered the first-line candidate for the treatment of oral and pharyngeal candidiasis. The efficacy of antifungal therapy for oral candidiasis is related to the time period the concentration of drug is above the minimum inhibitory concentration (MIC), which effect can be achieved locally in the mouth using buccal bioadhesive controlled release devices unlike existing conventional formulations (5).
Clotrimazole (CTZ) is the first line broad-spectrum antifungal agent that has been extensively used for the prophylaxis and treatment of oral and vaginal candidiasis (6). It is known to be very effective locally and only a small percentage of the drug applied to the oral mucosa can be detected in the serum or urine (7). Presently, for the topical treatment for oral candidiasis, clotrimazole (CTZ) is available only in the form of troche (a common brand is Mycelex® troche, Miles Pharma, USA) which is required to be taken three to five times a day for 14 days. Therefore, there is a need for the development of CTZ buccal bioadhesive controlled release formulation.
Recent years have seen an increasing interest in the development of novel buccal bioadhesive dosage forms. These are useful for both for systemic delivery of drugs, as well as for local targeting of drugs to a particular region in the body (8,9). A wide range of polymers of synthetic, semi synthetic and natural origin like carbopol, polycarbophil, sodium carboxymethylcellulose (SCMC), hydroxypropylmethylcellulose, chitosan and xanthan gum have been described for the formulation of bioadhesive systems but none of these polymer possess all the characteristics of an ideal polymer (nontoxic, nonirritant, strong non covalent adhesion, sustained release, stable and cheap) for a bioadhesive drug delivery system. Carbopols are excellent bioadhesives but with potential mucosal irritating character (10). Irritant properties of carbopol 974P (CP 974P) can be reduced by combining it with other non-irritant bioadhesive polymers like SCMC.
Therefore, the present study was aimed to design and develop buccal bioadhesive films of CTZ which would maintain the salivary concentration of the drug above the MIC against C. albicans for a prolonged period of time.
MATERIALS AND METHODS
Materials
Clotrimazole was obtained as gift sample from Amoli Organics Pvt. Ltd., Mumbai, India. Sodium carboxy methyl cellulose (S.D. Fine Chemicals, Mumbai, India), carbopol 974 P (Lupin Research Park, Pune, India), sabouraud dextrose agar (SDA) with chloramphenicol (Hi-Media, Mumbai, India) and glycerol (Qualigens Fine Chemicals, Mumbai, India) were used. All other chemicals were of analytical grade.
Preparation of the Buccal Bioadhesive Films
Doubled distilled water (DDW), used in the preparation of polymeric gel, was degassed under vacuum to avoid the formation of air bubbles. Weighed amount of CP 974P was added to one-third portion of the required DDW and kept undisturbed until a clear solution was formed. Then it was stirred for 1 h. CLZ was dissolved in a minimum volume of ethanol and added to SCMC contained in a dry beaker. The remaining two-third portion of DDW was added to the above mixture with stirring to form a homogeneous dispersion. The CP 974P solution and required volume of glycerol were added to the dispersion of SCMC and stirred for 3 h (Table I). The gel thus obtained was kept overnight undisturbed under refrigeration to ensure the formation of clear, bubble-free gel which was finally poured on a borosilicate glass mould (10 × 10 × 1.5 cm), allowed to settle and dried under convective flow of hot air at a temperature of 40–45 °C for 16–20 h till a flexible film was formed. After drying, the films were cut into smaller pieces of 1 × 1 cm sizes, wrapped in aluminum foil and stored in glass containers were preconditioned at room temperature and relative humidity of 60%.
Table I.
Formulae for Buccal Bioadhesive Films of CTZ
| Batch code | Amount of CLZ (mg/cm2 of the film) | Polymer concentration (% w/v of gel) | Ratio of SCMC to CP 974P | Glycerol concentration (% v/v of gel) |
|---|---|---|---|---|
| S1 | 1.5 | 1.25 | 100:0 | 1.5 |
| S2 | 1.5 | 1.25 | 95:05 | 1.5 |
| S3 | 1.5 | 1.25 | 90:10 | 1.5 |
| S4 | 1.5 | 1.25 | 80:20 | 1.5 |
| S5 | 1.5 | 1.25 | 70:30 | 1.5 |
Weight Uniformity
The weight of each of ten randomly selected films from every formulation batch was determined by using an electronic balance (Adair Dutt & Co, Kolkata, India) (11).
Thickness Testing
The thickness of ten randomly selected films from every formulation batch was determined using a standard screw gauge (11).
Folding Endurance
Folding endurance was determined by repeatedly folding the film at the same place till it broke or folded up to 300 times (11).
Drug Content Uniformity
Uniformity of drug content was determined according to the following procedure. Ten randomly selected films of each formulation batch were weighed accurately and dissolved in 10 ml of ethanol. Of this CTZ solution, 0.5 ml was transferred into a 100 ml volumetric flask containing 20 ml of PEG-400, and stirred continuously for 8 h on a magnetic stirrer. The volume was made up to 100 ml with phosphate buffer (pH 6.8) and the absorbances were measured in UV/Vis spectrophotometer at 260 nm (Jasco 7800, UV/Vis Spectrophotometer, Tokyo, Japan). Concentrations of CTZ were calculated from a standard calibration curve of CTZ in phosphate buffer (pH 6.8) containing 20% PEG-400 without interferences of excipients.
Microenvironment pH
The microenvironment pH of the prepared buccal bioadhesive CTZ films was determined to evaluate the possible irritation effects on the mucosa. The films were left to swell in 5 ml of distilled water (pH 6.8) in small beakers, and the pH was measured at time intervals of 2, 4, and 6 h by placing the electrode in contact with the microenvironment of the swollen films. The average pH of five determinations was reported.
Swelling Studies of Buccal Bioadhesive CTZ Films
The swelling index of the prepared buccal bioadhesive CTZ films was determined by weighing five films and recording their weights before placing them separately in weighed beakers. The total weight was recorded (W1). Four milliliters of phosphate buffer (pH 6.8) containing 20% PEG-400 was added to each beaker and then placed in an incubator at 37 ± 0.5 °C. At time intervals of 0.5, 1,1.5, 2, 3, 4, 5 and 6 h, excess water was carefully removed, and the swollen films were weighed (W2) (12). The experiment was repeated three times, and the average W1 and W2 were reported. The swelling index was determined from the formula:
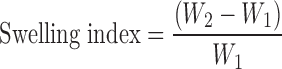 |
1 |
Moisture Absorption
Moisture absorption (MA) study was performed by a modified method of Kanig and Goodman (13). Accurately weighed preconditioned films (W0) were placed in a constant humidity chamber (containing a saturated solution of ammonium chloride which gives a relative humidity of 79.5%) set at room temperature. Films were weighed (Wt) at an interval of 1, 3 and 7 days.
Percent MA was calculated using the following equation:
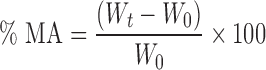 |
2 |
Vapor Transmission
A modification of the method used by Kanig and Goodman (13) was employed for the determination of vapor transmission from the film. Glass-bottle (length = 3.7 cm, internal diameter = 1.3 cm) filled with 2 g anhydrous calcium chloride and an adhesive (Quickfix®) spread across its rim, was used in the study. The film was fixed over the adhesive and the assembly was placed in a constant humidity chamber maintained at 37 ± 2 °C. The difference in weight after 24 h and 1 week was calculated. Vapor transmission rate was obtained as follows:
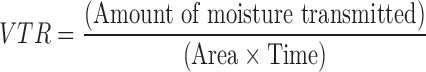 |
3 |
Mechanical Characterization of the Films
Mechanical parameters, tensile strength and elongation at break were calculated from the load time profiles of the films using INSTRON® tensile tester. Upper and lower grips of the sample with a gauge length of 5 × 1 cm, were attached to the crosshead and the base plate respectively in such a way that the former was located exactly 5 cm above the latter. The crosshead was moved upwards at a speed of 1 cm/s. The force and elongation were measured when the film broke (14). Results were reported as the mean (±SD) of five replicates.
The following equations were used:
 |
4 |
 |
5 |
Bioadhesive Strength
The force required to detach the bioadhesive films from the mucosal surface was applied as a measure of the bioadhesive performance. The method of Parodi et al. (15) was slightly modified for measuring the bioadhesion strength of the films. The instrument is broadly composed of a modified two arm physical balance in which the right pan had been replaced by a formulation holding glass plate (10 × 5 cm) and counter balanced by a water collecting pan suspended to the left arm. The pan received a siphon tube from a 10 L bottle, which was kept at a high place in such a way that water head in the bottle always remains above the water collecting pan. The siphon tube bears a flow regulating device. Nylon thread was used to suspend both the glass plate and the pan. An acrylate tissue mounting stage (1.8 × 1.8 × 8 cm) was attached to the center of a glass beaker (16 cm diameter and 18 cm height). Glass beaker was filled with phosphate buffer (pH 6.8) to simulate in-vivo saliva conditions. A magnetic stirrer provided with temperature control was used to maintain the temperature of phosphate buffer (pH 6.8) in glass dish at 37 ± 0.5 °C. A piece of rabbit intestinal mucosa, 3 cm long, was tightly secured on the upper surface of the acrylate tissue mounting stage with thread. Films were fixed on the centre of the formulation holding glass plate with an adhesive (Fevi Quick®). The exposed film surface was moistened with phosphate buffer (pH 6.8) and left for 30 s for initial hydration and swelling. Then glass plate (with the film) was kept on the mucosal tissue secured on the tissue mounting stage in such a way that films completely remained in contact with mucosa. The whole assembly was kept undisturbed for 3 min (preload time) to establish the adhesion between the film and mucosal tissue. The glass plate (weight 50 g) itself acted as a preload. After the preload time, water collecting pan was suspended to the left arm and water was added in it, by the siphon tube, at a constant rate of 200 drops per minute until detachment of the film from mucosal surface took place. A support was kept under the water collecting pan to hold it at the time of detachment. Weight of water collected in the pan at the time of detachment was measured. The experiment was performed in triplicate.
Residence Time
The in-vitro residence time was determined using a locally modified USP disintegration apparatus (Disintegration tester, Veego Instruments Corporation, Mumbai, India), based on the apparatus applied by Nafee et al. (11) and Nakamura et al. (16). The medium was composed of 500 mL phosphate buffer (pH 6.8) in 1 L beaker and maintained at 37 ± 0.5 °C. A segment of rabbit intestinal mucosa, 3 cm long, was glued on the inside curved surface of 1 L beaker above the level of 500 mL phosphate buffer (pH 6.8). A glass cylinder (100 mL) was vertically fixed to the apparatus. The bioadhesive film was hydrated from one surface using phosphate buffer (pH 6.8) and then the hydrated surface was brought into contact with the mucosal membrane. The glass cylinder was vertically fixed to the apparatus and allowed to move up and down so that the film was completely immersed in the buffer solution at the lowest point and was out at the highest point. The time necessary for complete erosion or detachment of the tablet from the mucosal surface was recorded (mean of triplicate determinations).
In-vitro Drug Release Studies
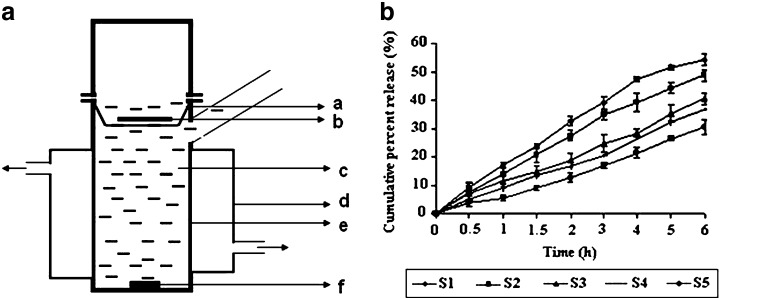
CTZ released from the prepared buccal bioadhesive CLZ films was determined by introducing single film in modified Franz diffusion cell {(External diameter 3.0 cm, internal diameter 2.8 cm, total height of the apparatus 8.0 cm, height of the receptor compartment 5.0 cm with a hat shaped stainless steel wire mesh basket for placing the films (2.6 cm diameter and 1 cm height)} having 30 mL of phosphate buffer (pH 6.8) containing 20% PEG-400 in receptor compartment (Fig. 1a). The receptor compartment maintained at 37 ± 0.5 °C was continuously stirred at 100 rpm. Samples of 3 mL were withdrawn at predetermined time intervals of over 8 h and replaced with equal volumes of the dissolution medium equilibrated at the same temperature. Drug concentration of the withdrawn samples was analyzed after filtration (0.45 μm Millipore filter) by UV/Vis Spectrophotometer at 260 nm (Jasco 7800, UV/Vis Spectrophotometer, Tokyo, Japan). All experiments were carried out in triplicate. Sink conditions were maintained throughout the study.
Fig. 1.
a, a Wire mesh, b buccal bioadhesive film, c phosphate buffer pH 6.8 containing 20% PEG-400 (volume of 30 ml), d water bath, e Franz diffusion cell, f small magnetic bead; b release profiles of CTZ from different buccal bioadhesive films in phosphate buffer (pH 6.8) containing 20% PEG-400 (n = 3)
Antifungal Efficacy of Buccal Bioadhesive Films
Preparation of the Agar Plates
The agar plates used in this study were prepared by dissolving SDA 65 g in 1 L of distilled water and sterilized by autoclaving (at 15 lb pressure and 121 °C) for 15 min. The agar solution was poured into sterile Petri dishes. The agar plates are then allowed to cool and solidify at room temperature; then they were inoculated (cultured) with C. albicans by using a sterile swab (17).
Agar Diffusion Assay of CTZ Films
Antifungal efficacy of the selected buccal bioadhesive film of CTZ (Batch S2) was determined by subjecting the aliquots of in-vitro drug release studies to disc agar diffusion assay (17,18). Aliquots of in-vitro drug release samples were collected at 0.5, 1, 2, 3, 4, 5 and 6 h.
A 0.1 mL of each sample was carefully pipetted into uniformly spaced 7 mm diameter wells of the agar plates. These plates were allowed to prediffuse for 2 h at room temperature and then incubated for 24 h. The diameter (millimeter) of the growth inhibition zone surrounding each agar well inoculated with C. albicans was measured, and the concentrations of CTZ were determined from the standard calibration curve constructed under identical condition, ranging from 5 to 80 μg/mL (19). The mean of three determinations of each sample was determined.
Statistical Analysis
The results obtained were subjected to statistical analysis using a computer program Sigma Stat® for one-way analysis of variance (ANOVA; p < 0.05).
RESULTS AND DISCUSSION
Physicochemical Characteristics of CTZ Buccal Bioadhesive Films
The bioadhesive buccal films containing CTZ were successfully prepared by solvent casting technique. Physical characteristics of the films are summarized in the Table II. The films from all the batches were translucent and flexible with the thickness ranging from 0.39 to 0.42 mm. The mass of films ranged from 49 to 52 mg. Drug content uniformity of films ranged from 1.43 to 1.48 mg. Low SD in thickness, weight measurement and drug content data reflected no significant difference within the batch.
Table II.
Physicochemical characteristics of the prepared buccal bioadhesive films of CTZ
| Batch code | Thickness, mm (mean ± SDa) | Weight, mg (mean ± SDa) | Drug content, mg (mean ± SDa) | Folding endurance | Microenvironment pH (mean ± SDb) |
|---|---|---|---|---|---|
| S1 | 0.39 ± 0.09 | 50 ± 1.07 | 1.48 ± 0.10 | >300 | 7.16 ± 0.12 |
| S2 | 0.41 ± 0.07 | 52 ± 0.95 | 1.44 ± 0.04 | >300 | 6.83 ± 0.09 |
| S3 | 0.39 ± 0.10 | 49 ± 1.73 | 1.46 ± 0.09 | >300 | 6.62 ± 0.16 |
| S4 | 0.42 ± 0.14 | 53 ± 2.19 | 1.43 ± 0.12 | >300 | 6.23 ± 0.11 |
| S5 | 0.41 ± 0.08 | 51 ± 1.44 | 1.47 ± 0.07 | >300 | 5.84 ± 0.19 |
a n = 10; standard deviation for ten determinations
b n = 5; standard deviation for five determinations
The microenvironment pH of different batches decreased with the increasing concentration of CP 974P due to its acidic nature (8). The decrease in pH may lead to mucosal irritation. Folding endurance was found to more than 300 for each case, indicative of reasonable flexibility of the films.
Swelling Index Studies
The swelling state of the polymer (in the formulation) was reported to be crucial for its bioadhesive behaviour. Adhesion occurs shortly after the beginning of swelling but the bond formed between mucosal layer and polymer is not very strong. The adhesion will increase with the degree of hydration until a point where over hydration leads to an abrupt drop in adhesive strength due to disentanglement at the polymer/tissue interface (14).
The swelling profiles of different batches of the films are shown in Table III. These profiles indicate the uptake of water into the film, producing an increase in weight. The swelling index of the prepared buccal bioadhesive films showed swelling rates in the order: S1 > S2 > S3 > S4 > S5, indicating that as the concentration of CP 974P was increased, the swelling index decreased. The maximum swelling was attained in 4 h for S1 and 5 h for S2; after which it decreased. The reason being that the rate of swelling is higher initially than the rate of erosion but after attaining maximum swelling index, erosion becomes predominant. S3, S4 and S5 swelled very slowly after 4 h and reached to plateau within 6 h; showing no visible sign of erosion even after 6 h.
Table III.
Swelling index of buccal bioadhesive films of CTZ at different time intervals
| Time (h) | Swelling index (mean ± SDa) | ||||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | |
| 0.5 | 19.61 ± 0.64 | 18.03 ± 3.43 | 17.24 ± 2.52 | 16.13 ± 1.65 | 14.05 ± 2.55 |
| 1 | 23.63 ± 1.68 | 22.51 ± 1.96 | 22.05 ± 1.76 | 19.52 ± 2.64 | 17.95 ± 2.44 |
| 1.5 | 26.15 ± 0.45 | 25.56 ± 2.58 | 24.22 ± 1.71 | 21.67 ± 0.48 | 19.86 ± 1.48 |
| 2 | 28.83 ± 2.57 | 27.05 ± 0.76 | 25.85 ± 2.85 | 22.19 ± 1.49 | 21.26 ± 0.49 |
| 3 | 29.71 ± 0.65 | 29.31 ± 1.33 | 28.41 ± 1.68 | 24.50 ± 3.68 | 23.51 ± 3.78 |
| 4 | 30.44 ± 0.61 | 30.94 ± 0.65 | 30.61 ± 0.98 | 26.83 ± 2.41 | 24.73 ± 0.81 |
| 5 | 28.85 ± 1.54 | 31.55 ± 1.61 | 31.34 ± 1.46 | 27.43 ± 1.64 | 24.97 ± 1.04 |
| 6 | 28.02 ± 0.84 | 30.73 ± 1.09 | 31.92 ± 1.54 | 28.06 ± 1.91 | 25.07 ± 2.91 |
a n = 5; standard deviation for five determinations
Moisture Absorption and Vapor Transmission Study
Tables IV and V show the percentage of moisture absorbed and moisture vapor transmission rate from the prepared films at different time intervals. The percent moisture absorbed decreased while vapor transmission rate increased with decreasing concentration of SCMC. There was no significant difference between the films S1 and S2, S3 and S4, S3 and S4, and S4 and S5 (p > 0.05, one way ANOVA), while the difference was significant between remaining possible pairs of formulations (p < 0.05, one way ANOVA). Similar moisture absorption characteristics were observed for the films S1 and S2. Percent moisture absorbed increased as the period of exposure to the humidity condition was increased up to 3 days. From the day 3 to 7, there was no significant (p > 0.05, one way ANOVA) absorption of moisture by the films.
Table IV.
Percent moisture absorbed by the films at different time intervals
| Batch code | Percent moisture absorbed (Mean ± SDa) | ||
|---|---|---|---|
| Day 1 | Day 3 | Day 7 | |
| S1 | 24.35 ± 1.33 | 28.07 ± 0.95 | 28.34 ± 1.08 |
| S2 | 24.15 ± 1.02 | 27.12 ± 0.89 | 27.12 ± 1.15 |
| S3 | 22.64 ± 2.12 | 24.45 ± 0.98 | 24.87 ± 1.42 |
| S4 | 21.22 ± 1.71 | 23.07 ± 0.83 | 23.07 ± 0.95 |
| S5 | 20.81 ± 1.20 | 22.64 ± 1.20 | 22.46 ± 1.53 |
a n = 3; standard deviation for three determinations
Table V.
Vapour transmission through the films at different time intervals
| Batch code | Moisture vapour transmission, g cm−2 h−1 (mean ± SDa) | ||
|---|---|---|---|
| Day 1 | Day 3 | Day 7 | |
| S1 | 5.2 × 10−3 ± 1.56 × 10−3 | 5.8 × 10−3 ± 3.59 × 10−3 | 6.5 × 10−3 ± 1.78 × 10−3 |
| S2 | 6.4 × 10−3 ± 3.54 × 10−3 | 6.3 × 10−3 ± 1.24 × 10−3 | 6.6 × 10−3 ± 3.74 × 10−3 |
| S3 | 7.0 × 10−3 ± 2.36 × 10−3 | 6.9 × 10−3 ± 2.36 × 10−3 | 6.8 × 10−3 ± 2.34 × 10−3 |
| S4 | 8.3 × 10−3 ± 1.84 × 10−3 | 7.8 × 10−3 ± 2.68 × 10−3 | 7.5 × 10−3 ± 2.14 × 10−3 |
| S5 | 8.2 × 10−3 ± 2.61 × 10−3 | 7.8 × 10−3 ± 3.27 × 10−3 | 7.8 × 10−3 ± 1.69 × 10−3 |
a n = 5; standard deviation for five determinations
Mechanical Properties
Table VI shows the mechanical properties of the prepared drug loaded films. The results shows that increase in CP 974P content reduced both the tensile strength and elongation break significantly, indicative of a weaker and less elastic, less flexible films. The films with increased concentration of CP 974P were more opaque indicative of more unsolubilized drug which may be physically interrupting the polymeric matrix thus resulting into the formation of hard and brittle films.
Table VI.
Mechanical properties of the prepared bioadhesive buccal films of CTZ
| Batch code | Tensile strength (kg mm−2; mean ± SDa) | Elongation at break (%mm−2; mean ± SDa) | ||
|---|---|---|---|---|
| Films with 1.5% glycerol | Films with 0.5% glycerolb | Films with 1.5% glycerol | Films with 0.5% glycerolb | |
| S1 | 2.23 ± 0.13 | 4.78 ± 0.18 | 49.64 ± 1.25 | 22.35 ± 0.16 |
| S2 | 2.32 ± 0.19 | 4.54 ± 0.21 | 48.95 ± 2.05 | 24.58 ± 2.46 |
| S3 | 1.86 ± 0.15 | 3.13 ± 0.14 | 39.08 ± 1.46 | 18.87 ± 2.14 |
| S4 | 1.05 ± 0.18 | 2.42 ± 0.25 | 32.60 ± 1.97 | 14.47 ± 1.52 |
| S5 | 0.86 ± 0.09 | 1.96 ± 0.11 | 25.67 ± 1.24 | 12.54 ± 1.36 |
a n = 5; standard deviation for five determinations
bFilms with 0.5% v/v glycerol concentration was prepared only to study mechanical properties
Increase in glycerol concentration resulted in more elastic but weaker films. Commonly, addition of a plasticizer to a polymeric system lowers the glass transition temperature of that system, rendering it softer and more flexible.
Bioadhesion Force and Residence Time
Bioadhesion force and residence time of the prepared films on rabbit intestinal mucosa as a function of CP 974P and SCMC ratio have been shown in Table VII. Films containing 5% CP 974P possessed the highest bioadhesive force and residence time; increase in CP 974P content beyond this proportion resulted into a decrease in bioadhesion. But no statistically significant difference was found in bioadhesive force between S2 and S3 and among S3, S4 and S5 (p > 0.05, one way ANOVA). For remaining of the possible pairs, the difference was found significant (P < 0.05, one-way ANOVA).
Table VII.
Bioadhesion force and residence time of prepared buccal bioadhesive films of CTZ
| Batch code | Bioadhesion force (g; mean ± SDa) | Residence time (h; mean ± SDa) |
|---|---|---|
| S1 | 347 ± 14.3 | 3.26 ± 0.35 |
| S2 | 519 ± 24.7 | 6.75 ± 0.15 |
| S3 | 493 ± 16.1 | 6.18 ± 0.27 |
| S4 | 439 ± 21.9 | 4.84 ± 0.11 |
| S5 | 405 ± 18.7 | 4.57 ± 0.24 |
a n = 3; standard deviation for three determinations
With the ratio of 70:30 to 80:20 of SCMC and CP 974P, there occurs a high polymer chain entanglement and complexation; thus leading to reduced availability of free functional groups of polymers to substrate and consequent low bioadhesion (20). Moreover, bioadhesive property of CP 974P is known to decrease beyond pH 6 (21). Our study was performed in phosphate buffer (pH 6.8), which may be another explanation for the low bioadhesion.
Increase of CP 974P content in the formulations (up to 30% of total polymer concentration) results in significant decrease in residence time (P < 0.05, one-way ANOVA). S1 which did not contain CP 974P showed the shortest residence time. This occurred due to the erosion of the film S1 and not due to its dislodgment from the mucosal tissue. Though it had shown a mean residence time of 3.26 h but it became a loose mass of polymer even after 2.5 h, so unsuitable for prolonged intra-oral delivery of CTZ.
In-vitro Drug Release Studies
Figure 1b shows release profiles of the bioadhesive buccal films. The rate and extent of drug release decreased (from S1 to S5) as the concentration of CP 974P increased in SCMC based films. Sustained release was observed in each case which may be attributed to the low solubility of CLZ along with highly coiled network of CP 974P. The difference in cumulative percent release (CPR) of different formulations was found significant except between S3 and S4 (P < 0.05, one way ANOVA). S1 batch showed the highest CPR (56.48 ± 1.83) which may be attributed to the higher swelling ability of SCMC. Pronounced swelling along with erosion of SCMC matrix allowed the drug to diffuse at a faster rate; however it cannot be utilized to prolong the drug release. S2 batch also showed CPR of 49.24 ± 1.55 which is less than S1 batch. The presence of CP 974P decreased the drug release from the S2 batch.
Drug Release Kinetics
To examine the release mechanism of CTZ from the prepared buccoadhesive films, the results were analyzed according to the following equation (22–23).
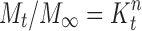 |
6 |
where 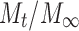 is the fractional drug released at time t, k is a kinetic constant incorporating structural and geometrical characteristics of the drug/polymer system (device), and n is the diffusional exponent that characterizes the mechanism of drug release. For non-Fickian release, the n value falls between 0.5 and 1.0 (0.5 < n < 1.0), whereas in the case of Fickian diffusion, n = 0.5; for zero-order release (case II transport), n = 1, and for super case II transport, n > 1 (15,24). The values of n as estimated by linear regression of log
is the fractional drug released at time t, k is a kinetic constant incorporating structural and geometrical characteristics of the drug/polymer system (device), and n is the diffusional exponent that characterizes the mechanism of drug release. For non-Fickian release, the n value falls between 0.5 and 1.0 (0.5 < n < 1.0), whereas in the case of Fickian diffusion, n = 0.5; for zero-order release (case II transport), n = 1, and for super case II transport, n > 1 (15,24). The values of n as estimated by linear regression of log 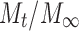 vs. log (t) of different formulations are shown in Table VIII. The n values were between 0.5 and 1.0 for the release of CTZ from all the film formulations except for S1, indicating non-Fickian release kinetics, which is indicative of drug release mechanisms involving a combination of both diffusion and chain relaxation mechanisms (18,25,26). For S1, value of n was found to be 1.019, indicative of zero-order release by erosion.
vs. log (t) of different formulations are shown in Table VIII. The n values were between 0.5 and 1.0 for the release of CTZ from all the film formulations except for S1, indicating non-Fickian release kinetics, which is indicative of drug release mechanisms involving a combination of both diffusion and chain relaxation mechanisms (18,25,26). For S1, value of n was found to be 1.019, indicative of zero-order release by erosion.
Table VIII.
Linear correlation coefficient (r), determination coefficients (r 2), kinetic release constants (K), and diffusion exponents (n) after fitting the release data of CTZ to the simple power law (log M t / M ∞ vs. log t)
| Batch code | r | r 2 | K (h −n ) | n a |
|---|---|---|---|---|
| S1 | 0.9964 | 0.9928 | 10.9197 | 1.0190 |
| S2 | 0.9992 | 0.9984 | 10.5900 | 0.8650 |
| S3 | 0.9912 | 0.9835 | 10.3371 | 0.7020 |
| S4 | 0.9921 | 0.9842 | 8.6796 | 0.8078 |
| S5 | 0.9743 | 0.9492 | 6.0030 | 0.8953 |
n a The diffusion release exponent, indicative of the release mechanism; n = 0.5 in case of the diffusion mechanism; n = 1 for zero-order release, and for super case II transport, n > 1. n lies between 0.5 and 1.0 (0.5 < n < 1) for non-Fickian (anomalous) release and n < 0.45 for Fickian release mechanism
The S1 batch showed higher release rate (K = 10.91) but without any bioadhesion character. The next higher release rate (K = 10.59) was shown by S2 batch containing 5% CP 974P (Table VIII). This batch showed maximum residence time and maximum bioadhesion force.
It was concluded that formulations containing 5% CP 974P (Batch S2) characterized by moderate swelling, a maximum residence time, a maximum bioadhesion force as well as faster rate of in-vitro drug release which is favorable for a poorly soluble drug. Batch S3–S5 containing high concentration of CP 974P showed less bioadhesive force and in-vitro residence time when compared with batch S2. Batch S2 was thus selected for investigation of further microbiological studies.
Antifungal Efficacy of Selected Buccal Bioadhesive Film
Table IX show the antifungal activity of the aliquot sample against C. albicans. The drug released from the selected film S2 was able to inhibit the growth of C. albicans for 6 h. A maximum growth inhibition zone of 11.6 mm was obtained with the aliquot from 6 h dissolution sample. There was no inhibition of growth for the first dissolution sample which may be due to the CTZ concentration below the MIC. From the standard calibration curve of CTZ agar diffusion assay, the growth inhibition zone of 6 h dissolution sample of batch S2 corresponds to 48.61% of CTZ release which correlated well with dissolution studies data.
Table IX.
Diameter of zone of inhibition for in-vitro release samples of selected buccal bioadhesive film of CTZ (batch S2)
| S. No | Time of sampling (h) | Diameter of zone of inhibition (mm) (mean ± SDa) | Corresponding drug release (%) (mean ± SDa) |
|---|---|---|---|
| 1 | 0.5 | 0 | 0 |
| 2 | 1.0 | 2.3 ± 0.8 | 9.98 ± 1.09 |
| 3 | 1.5 | 3.6 ± 0.5 | 15.26 ± 1.53 |
| 4 | 2.0 | 4.6 ± 0.8 | 19.86 ± 0.91 |
| 5 | 3.0 | 6.6 ± 0.5 | 27.94 ± 1.54 |
| 6 | 4.0 | 8.6 ± 0.5 | 34.73 ± 1.04 |
| 7 | 5.0 | 10.3 ± 0.8 | 42.06 ± 0.83 |
| 8 | 6.0 | 11.6 ± 0.8 | 48.61 ± 0.95 |
a n = 3; standard deviation for three determinations
CONCLUSIONS
All the prepared CTZ buccal bioadhesive films gave a reasonable in-vitro residence time (3.26–6.75 h), which is important for prolonging the contact time of the drug with the buccal mucosa, thus improving the overall therapy of oral candidiasis. Increasing CP 974P concentration resulted in decreasing the swelling index and microenvironment pH. Percent moisture absorbed decreased while vapor transmission rate increased with increasing the concentration of CP 974P. Increase in CP 974P content reduced both the tensile strength and elongation break. Bioadhesive force and the in-vitro residence time decreased upon increasing the concentration of CP 974P beyond 5%. The prepared buccal bioadhesive films provided a controlled and prolonged in-vitro release of CTZ. Duration of growth inhibition (antifungal activity) of buccal bioadhesive films of CTZ was prolonged up to 6 h. This would be important for better patient compliance because of the decrease in the frequency of administration.
Present study showed the in-vitro efficacy of buccal bioadhesive films of CTZ against C. albicans for prolonged period of time. In future, in-vivo studies will be carried out to assess the efficacy of buccal bioadhesive films of CTZ in comparison with marketed products. The in-vivo buccal bioadhesion and in-vivo buccal CTZ concentration released from buccal films will be studied in healthy human volunteers (11).
Contributor Information
S. Singh, Phone: +91-542-2315871, FAX: +91-542-2368428, Email: drsanjaysingh@rediffmail.com
M. S. Muthu, Email: muthubits@rediffmail.com
References
- 1.Ellepola A. N., Samaranayake L. P. Antimycotic agents in oral candidiasis: an overview:1. Clinical variants. Dent. Update. (2000);27:111–116. doi: 10.12968/denu.2000.27.3.111. [DOI] [PubMed] [Google Scholar]
- 2.Albougy H. A., Naidoo S. A systematic review of the management of oral candidiasis associated with HIV/AIDS. SADJ. (2002);57:457–466. [PubMed] [Google Scholar]
- 3.Pienaar E. D., Young T., Holmes H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst. Rev. (2006);3:CD003940. doi: 10.1002/14651858.CD003940.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. (1992);14(suppl 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 5.Roey J. V., Haxaire M., Kamya M., Lwanga I., Katabira E. Comparative efficacy of topical therapy with a slow release mucoadhesive buccal tablet containing miconazole nitrate versus systemic therapy with ketoconazole in HIV-positive patients with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. (2004);35:144–150. doi: 10.1097/00126334-200402010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Martin M. V. Antifungal agents. In: Samaranayake L. P., MacFarlane T. W., editors. Oral Candidiasis. London: Butterworth and Co; 1990. pp. 238–259. [Google Scholar]
- 7.Plempel M., Bartmann K., Buchel K. H., Regel E. BAY b 5097, a new orally applicable antifungal substance with broad-spectrum activity. Antimicrob. Agents Chemother. (1969);69:271–274. [PubMed] [Google Scholar]
- 8.Bouckaert S., Remon J. P. In-vitro bioadhesion of a buccal, miconazole slow release tablet. J. Pharm. Pharmacol. (1993);45:504–507. doi: 10.1111/j.2042-7158.1993.tb05588.x. [DOI] [PubMed] [Google Scholar]
- 9.Save T., Venkitachalam P. Buccoadhesive tablets of nifedipine in standardization of a novel buccoadhesive erodible carrier. Drug Dev. Ind. Pharm. (1994);20:3005–3014. doi: 10.3109/03639049409041964. [DOI] [Google Scholar]
- 10.Ahuja A., Khar R. K., Ali J. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. (1997);23:489–515. doi: 10.3109/03639049709148498. [DOI] [Google Scholar]
- 11.Nafee N. A., Ismail F. A., Boraie N. A., Mortada L. M. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int. J. Pharm. (2003);1–2:1–14. doi: 10.1016/S0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal V., Mishra B. Development and biopharmaceutical properties of buccoadhesive compacts of pentazocine. Drug Dev. Ind. Pharm. (1999);25:701–709. doi: 10.1081/DDC-100102229. [DOI] [PubMed] [Google Scholar]
- 13.Kanig J. L., Goodman H. Evaluative procedures for film forming materials used in pharmaceutical applications. J. Pharm. Sci. (1962);51:77–83. doi: 10.1002/jps.2600510115. [DOI] [PubMed] [Google Scholar]
- 14.Peh K. K., Wong C. F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharmaceut. Sci. (1999);2:53–61. [PubMed] [Google Scholar]
- 15.Parodi B., Russo E., Caviglioli G., Cafaggi S., Bignardi G. Development and characterization of a buccoadhesive dosage form of oxycodone hydrochloride. Drug Dev. Ind. Pharm. (1996);22:445–450. doi: 10.3109/03639049609069353. [DOI] [Google Scholar]
- 16.Nakamura F., Ohta R., Machida Y., Nagai T. In-vitro and in vivo mucoadhesion of some water soluble polymers. Int. J. Pharm. (1996);134:173–181. doi: 10.1016/0378-5173(95)04416-7. [DOI] [Google Scholar]
- 17.Khanna R., Agarwal S. P., Ahuja A. Mucoadhesive buccal tablets of clotrimazole for oral candidiasis. Drug Dev. Ind. Pharm. (1997);23:831–837. doi: 10.3109/03639049709150554. [DOI] [Google Scholar]
- 18.Mohammed F. A., Khedr H. Preparation and in vitro/in vivo evaluation of the buccal bioadhesive properties of slow-release tablets containing miconazole. Drug Dev. Ind. Pharm. (2003);29:321–337. doi: 10.1081/DDC-120018206. [DOI] [PubMed] [Google Scholar]
- 19.Holt R. J. Laboratory tests of antifungal drugs. J. Clin. Path. (1975);28:767–774. doi: 10.1136/jcp.28.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varshosaz J., Dehghan Z. Development and characterization of buccoadhesive nifedipine tablets. Eur. J. Pharm. Biopharm. (2002);54:135–141. doi: 10.1016/S0939-6411(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H. S., Park H., Kelly P., Robinson J. R. Bioadhesive polymer as platforms for oral controlled drug delivery II: synthesis and evaluation of some swelling water-insoluble bioadhesive polymers. J. Pharm. Sci. (1985);74:399–405. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 22.Deasy P. B., O’Neill C. T. Bioadhesive dosage form for peroral administration of timolol base. Pharm. Acta Helv. (1989);64:231–235. [PubMed] [Google Scholar]
- 23.Krosmeyer R. W., Gurny R., Doelkar E., Buri P., Peppas N. A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. (1987);15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 24.Shargel L., Andrew B. C. Applied biopharmaceutics and pharmacokinetics. New York: Prentice Hall, Appleton-Century-Crofts; 1992. [Google Scholar]
- 25.Ritger P. L., Peppas N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release. (1987);5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 26.Peppas N. A., Krosmeyer R. W. Hydrogels in medicine and pharmacy: properties and applications. Boca Raton: CRC; 1986. p. 109. [Google Scholar]