Abstract
P-glycoprotein (P-gp) is a membrane-bound transporter protein that is encoded by the human multidrug resistance gene MDR1 (ABCB1). P-gp recognizes a wide range of xenobiotics, is pivotal in mediating cancer drug resistance, and plays an important role in limiting drug penetration across the blood–brain barrier. MDR1 genetic variation can lead to changes in P-gp function and may have implications on drug pharmacokinetics. We have identified a novel MDR1GT1292-3TG (Cys431Leu) genetic variation through systematic profiling of subjects with leukemia. The cellular and transport function of this variation was investigated with recombinant human embryonic kidney cells expressing MDR1. Compared with the wild type, MDR1GT1292-3TG recombinant cells exhibited a lower drug resistance phenotype for a panel of chemotherapeutic agents. When compared with wild type, MDR1GT1292-3TG recombinant cells exposed exhibited a 75% decrease in IC50 for doxorubicin (162.6 ± 17.4 to 37.9 ± 2.6 nM) and a 50% decrease in IC50 for paclitaxel (155.7 ± 27.5 to 87.7 ± 9.2 nM), vinblastine (128.0 ± 15.9 to 65.9 ± 5.1 nM), and vincristine (593.7 ± 61.8 to 307.3 ± 17.0 nM). The effects of the Cys431Leu variation, due to MDR1GT1292-3TG nucleotide transition, on P-gp-dependent intracellular substrate accumulation appeared to be substrate dependent where doxorubicin, vinblastine, and paclitaxel exhibit an increased accumulation (p < 0.05), while verapamil and Hoechst33342 exhibit a decreased intracellular concentration compared with wild type (p < 0.05). Collectively, these data suggest MDR1GT1292-3TG variation of P-gp may reduce drug resistance and that subjects with this genotype undergoing chemotherapy with drugs that are transported by P-gp could potentially be more responsive to therapy than those with MDR1 wild-type genotype.
Key words: ABC transporter, drug resistance, genetic variation, MDR1, P-glycoprotein, polymorphism, transporter
INTRODUCTION
P-glycoprotein (P-gp), an efflux transporter and a member of the ATP binding cassette (ABC) transporter super-family, resides in the plasma membrane and expresses in both tumor cells and normal cells. A number of cancer cells over express P-gp and can increase transcription of MDR1 by drug and other compounds (1). The substrates of P-gp-mediated efflux transport include a wide range of natural compounds and lipophilic xenobiotics (2,3). The transport function of P-gp is driven by ATP hydrolysis; however, the stoichiometry of ATP to substrate ratio per transport cycle is yet to be confirmed.
P-gp is expressed on epithelial cells and exerts its largest effect on the pharmacokinetics of drugs in the intestinal mucosa, liver canalicular membrane, kidney proximal tubules, blood–brain barrier, and placenta (4,5). P-gp is believed to function as a protective barrier against many chemotherapeutic agents and toxic xenobiotics by reducing intracellular and transcellular substrate accumulation. This can be seen though decreased intestinal absorption, enhanced biliary excretion and renal tubular secretion, and limited drug distribution to the fetus and brain. In the case of P-gp expressed in the blood–brain barrier, significant reduction or abolishment of the P-gp function has been demonstrated in mice to significantly increase anti-HIV and cancer drug availability to the brain (6,7). Thus, sequence variations in MDR1 that potentiate significant change in transport function could affect the outcome of chemotherapy and the disposition of a broad range of xenobiotics.
We have previously reported the functional significance of the G1199A, G1199T, and G571A genetic variations using recombinant MDR1 cells expressing respective P-gp variants (8–10). MDR1G1199A and MDR1G1199T variations result in an amino acid substitution at amino acid Ser400 in the first ATP binding domain cytoplasmic loop. Ser400 resides next to the highly conserved Tyr401 and is part of the A-loop responsible for π–π stacking with the adenine base of ATP (11,12). When compared with wild-type MDR1, MDR1G1199A, resulting in a serine to asparagine transition, displayed greater resistance to vinblastine, vincristine, and paclitaxel with no significant difference with doxorubicin, while MDR1G1199T, a serine to isoleucine transition, decreased resistance to all of these drugs. MDR1G571A (Gly191Arg) is located in the third transmembrane (TM) domain of P-gp and decreased resistance to vinblastine, vincristine, and paclitaxel with no significant difference with doxorubicin.
The aim of the present study is to investigate the functional impact of a novel MDR1 variant on P-gp transport and drug resistance function. To do so, we have developed and characterized a set of stable recombinant P-gp-expressing cells. These cells were used to investigate the role of the GT1292-3TG novel nucleotide variation on P-gp functions.
MATERIALS AND METHODS
Chemicals and Drugs
Doxorubicin, paclitaxel (Sigma-Aldrich, St Louis, MO), vinblastine (Bedford Laboratories, Bedford, OH, USA), vincristine (Faulding, Paramus, NJ, USA), and topotecan (GlaxoSmithKline, Research Triangle Park, NC, USA) were diluted with culture medium for the cytotoxicity. Multidrug resistance protein inhibitor GF120918 [N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl) ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide] was kindly provided by GlaxoSmithKline. Rhodamine 123 (R123) was purchased from Sigma-Aldrich Co. Bodipy FL-vinblastine, Bodipy FL-verapamil, Paclitaxel-Oregon Green® 488, tissue culture media DMEM, trypsin–EDTA, and antibiotic solutions were supplied by Invitrogen Corp. (Carlsbad, CA, USA). Fetal bovine serum was obtained from Hyclone (Logan, UT, USA). Other reagents were of analytical grades or higher.
Patient MDR1 Sequencing
Blood or bone marrow samples were used to extract RNA, which was reverse transcribed to obtain MDR1 cDNA as previously described (13). The MDR1 nucleotide sequence for each subject (with no identifiable clinical information under an approved human subject protocol by the University of Washington) was determined by directly sequencing the 3.8-kb cDNA in both sense and anti-sense direction with an automated DNA sequencer based on Big-Dye 3.0 chemistry (Applied Biosystems, Foster City, CA, USA). The sequence data were assembled and analyzed with the Vector NT program. Patient demographics were previously described (8).
Cell Culture
Human embryonic kidney cells, HEK293 (HEK) with or without P-gp expression, were grown at 37°C in complete media consisting of DMEM medium (Invitrogen) supplemented with 10% (v/v) fetal calf serum, 1% (v/v) antibiotic–antimycotic, and 1% non-essential amino acid, and under 5% CO2.
Generation of MDR1wt and MDR1GT1292-3TG Plasmids
The method was similar to our previously reported method (8). Briefly, MDR1 cDNA was cloned into a linearized pcDNA-TA vector (Invitrogen) containing cytomegalovirus (CMV) and T7 promoters capable of transcription, also described previously (13). The mammalian expression plasmid containing the GT1292-3TG mutation was generated by site-directed mutagenesis (Stratagene, La Jolla, CA, USA). Details of this expression plasmid design to express P-gp in mammalian cells have been published (9). Clones were screened by restriction enzyme mapping, and the sequence was verified. The variant expression plasmid was designated as MDR1GT1292-3TG.
Isolation of Stable Recombinant Cells Expressing MDR1wt or MDR1GT1292-3TG
Ten million HEK cells were transfected with 10 μg of either MDR1wt and MDR1GT1292-3TG plasmid by electroporation performed at 250 V and 975 μF in a Gene Pulser® II (Bio-Rad Laboratories, Hercules, CA, USA). Subsequently, cells were kept at 4°C for 10 min before returning to the original cell culture conditions. These cells were exposed to increasing concentrations of G418 (Calbiochem, San Diego, CA, USA), a neomycin derivative, up to 800 μg/ml to select for cells that express neomycin resistance under the direction of the same plasmid vector. The G418 resistance cells were verified to have stable, high levels of P-gp expression and further reduced to two to four clones for final selection. The stable clone that consistently expressed P-gp or variants was picked and used for functional studies and validation.
Western Immunoblot Analysis
Western immunoblot analysis was used to characterize molecular weight and verify P-gp protein expression in the recombinant cells. Control and recombinant P-gp-expressed cells were pelleted and washed in PBS, followed by cell lysis in 1% NP-40 in water. The cell lysate was centrifuged at 400× gravity to pellet the nucleus and unbroken cells. Protein concentration was measured by a microplate assay protocol (DC Protein Assay, Bio-Rad Laboratories). The cell lysate was then placed in Lamelli sample buffer containing SDS and β-mercaptoethanol. A 4–20% gradient SDS-PAGE gel was loaded with 2.5 μg protein per well and ran at 130 V for 60 min. The transfer to a PVDF membrane was run for 90 min at 90 V according to instructions for Mini Trans-Blot® Electrophoretic Transfer Cell (Bio-Rad Laboratories). Non-specific binding sites on the membrane were blocked with 5% evaporated milk in TTBS buffer (0.1% Tween 20; 20 mM Tris–HCl, 0.9% NaCl, pH 7.6). Subsequently, the membrane was incubated with the F4 (Sigma) anti-P-gp monoclonal antibody, followed by a secondary horse reddish peroxidase (HRP)-conjugated goat anti-mouse IgG1 antibody. ECL-Plus reagent was used as a substrate, and blots were exposed to X-ray film to detect anti-P-gp reactive protein bands.
Rhodamine123 Efflux and Inhibition Assay
Recombinant cells expressing control and MDR1 variants were seeded into 12-well tissue culture plates at 1 × 106 cells per well and incubated at 37°C overnight. Medium was removed, and cells were preincubated with 0.5 mL of serum-free medium or medium containing 1 μM GF120918 for 5 min before replacing with 0.5 mL medium containing 1 μM R123 or 1 μM R123 plus 1 μM GF120918 and incubated for 30 min at 37°C. Medium was removed, and the plates were washed twice with warm PBS. One half milliliter of medium or medium containing 1 μM GF120918 was added, and efflux was performed at 37°C for 30 min. After extensive washing of the cells, cells were lysed with 0.5% deoxycholate (DOC). The intracellular R123 fluorescence (λex = 485 nm; λem = 535 nm) was measured on a Victor III multiplate reader (PerkinElmer, Waltham, MA, USA).
Drug Sensitivity Assessment
The method was the same as described previously (8). Briefly, cells were seeded out in 96-well plates and grown overnight at 37°C. Varying concentrations of doxorubicin, paclitaxel, vinblastine, vincristine, and topotecan were added to the cells in quadruplicate. Cells were incubated with the indicated drugs for 72 h at 37°C. The viability was measured using CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS) (Promega, Madison, WI, USA). The resultant effective concentration necessary for 50% cell growth inhibition (IC50) for each drug was estimated using a sigmoid Emax model in SigmaPlot. The statistics between each group were analyzed with a one-way ANOVA followed by a Tukey post test.
Drug Uptake
Cells were plated at a density of 5.5 × 105 cells/well in 500 μL in a 24-well plate (Corning) and grown overnight. The growth media were removed and replaced with 1 μM R123, 5 μM doxorubicin, 1 μM Paclitaxel-Oregon Green® 488, 1 μM Bodipy FL-vinblastine, 0.3 μM Bodipy FL-verapamil, or 0.4 μM Hoechst33342 in DMEM for 30 min at 37°C in the presence of 5% CO2. Cells were washed three times with PBS and lysed in 50 μL 0.5% DOC; 100 μL PBS was added to each well, and the fluorescence was read on a Victor III plate reader. Results were normalized for protein content and expressed as percentage of drug uptake in HEK control cells. The statistics between each group were analyzed with a one-way ANOVA followed by a Tukey post test.
Crude Membrane Preparation
Cells were grown in T175 flasks in DMEM + 10%FBS to 85% confluence. The cells were washed once with PBS. Cells were then washed with ice-cold hypotonic lysis buffer (10 mM Tris–HCl pH 7.5, 10 mM NaCl, 1 mM MgCl2, 1× protease inhibitor cocktail). The cells were then scraped off, incubated in lysis buffer for 10 min, broken by agitation, and centrifuged at 325×g for 5 min at 4°C to pellet the nucleus and unbroken cells. The supernatant containing the cell membrane was centrifuged at 20,000× gravity for 60 min at 4°C. The membrane pellet was resuspended in resuspension buffer (20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 50 mM sucrose, protease inhibitor) and pelleted again at 20,000× gravity for 60 min at 4°C. The resulting crude membrane pellet was resuspended in 0.2 mL resuspension buffer per T175. The Dc Protein Assay (Bio-Rad) was used to measure protein concentration.
ATPase Activity
In a 96-well plate, crude membrane (20 μg protein) was incubated in 90 μL assay buffer (50 mM Tris pH 7.4, 50 mM KCl, 5 mM sodium azide, 2 mM EGTA, 2 mM oubain, 2 mM DTT, 10 mM MgCl2, and 0.1% tween 20) with or without drug in the presence and absence of 300 μM sodium orthovanadate for 5 min at 37°C. The ATPase reaction was started by adding 10 μL of 50 mM MgATP for a 5 mM final concentration, and incubated for 45 min at 37°C. The reaction was stopped by addition of colorimetric phosphate detection solution (6% (w/v) SDS, 3% (w/v) ascorbic acid, 0.5 M HCl, and 0.5% (w/v) ammonium molybdate). After incubation for 10 min, unreacted molybdate was complexed with a solution containing 2% (w/v) Na3 citrate, 2% (w/v) Na m-arsenite, and 2% (v/v) acetic acid. Absorbance was measured at 720 nm.
RESULTS
Identification and Validation of the GT1292-3TG Variation in Leukemia Patients
As part of an effort to identify MDR1 genetic variations in leukemia (8), we sequenced the entire coding sequences of MDR1 from myeloblast and bone marrow cells collected from leukemia patients. We found a novel GT→TG transition at the 1292-3 nucleotide positions that predicted to produce a Cys→Leu modification of P-gp at amino acid 431. The novel variant 1292-3TG is found to have an estimated frequency of 1.35% and an RNA copy number of 1,126 ± 137 copies per nanogram of RNA. The patient carrying the GT1292-3TG variation did not have any other coding region variation within the MDR1 gene. The RNA copy number is significantly higher (p < 0.01) and roughly 2.5-fold higher than the wild-type samples (Table I).
Table I.
Allelic Frequency of MDR1 1292-3 Variants in Leukemia Subjects
| Sequence/variation | Amino acid variation | Estimated allelic frequency in leukemia patients | Copies per ng RNA |
|---|---|---|---|
| Wild type | Cys 431 | 0.9865 | 459 ± 293 |
| GT 1292-3 TG | Cys 431 Leu | 0.0135 | 1126 ± 137* |
The RNA from 74 leukemia patients was collected, and the MDR1 RNA transcripts were reverse transcribed into complementary DNA. These MDR1 DNA products were sequenced and analyzed to assign the allele for each individual subject. These data were validated by direct sequencing of DNA from the same subject, and were presented as overall allelic frequencies
*p < 0.01 compared with wild type
Development and Characterization of Recombinant Cells Expressing MDR1 and Variants
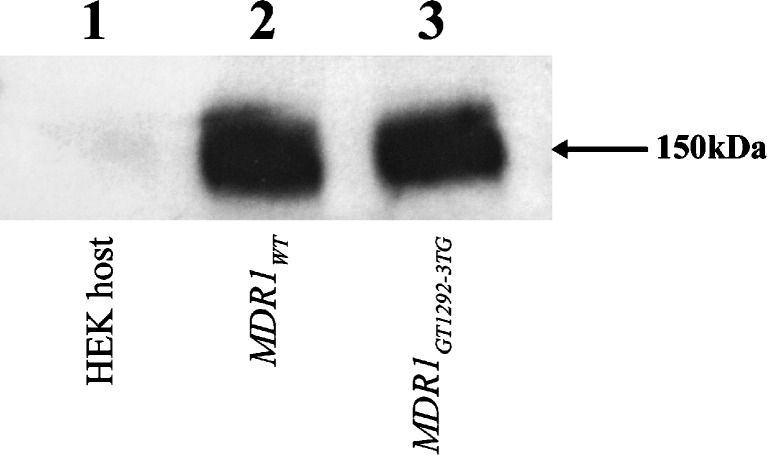
Following construction of the expression plasmid carrying MDR1 and its variants, the plasmids were transfected into the MDR1 negative mammalian host HEK cells. Cells carrying the MDR1GT1292-3TG gene with a high degree of expression similar to that of the previously developed wild-type expressing cells were expanded. Western blot was used to characterize the molecular weight of the protein and the degree of expression in MDR1wt and MDR1GT1292-3TG stable recombinant HEK cells. The apparent molecular weight of P-gp expressed in representative recombinant cells expressing MDR1wt and MDR1GT1292-3TG was recorded at approximately 150 kDa, and the level of P-gp expression was equivalent (Fig. 1). Western immunoblot analysis was also performed on the crude membrane fraction to ensure that the expressed P-gp protein was located on cell membrane.
Fig. 1.
Comparison of P-gp protein expression in recombinant MDR1 and host HEK cells. Total cell lysates from recombinant cell expressing MDR1 WT (lane 2), MDR1 GT1292-3TG (lane 3), or MDR1 negative HEK host (lane 1) were separated by gel electrophoresis, then blotted onto a PVFD membrane. The membrane was probed with the F4 anti-P-gp monoclonal antibody, developed with a ECL reagent, and exposed to X-ray film to detect anti-P-gp reactive protein bands at ~150 kDa
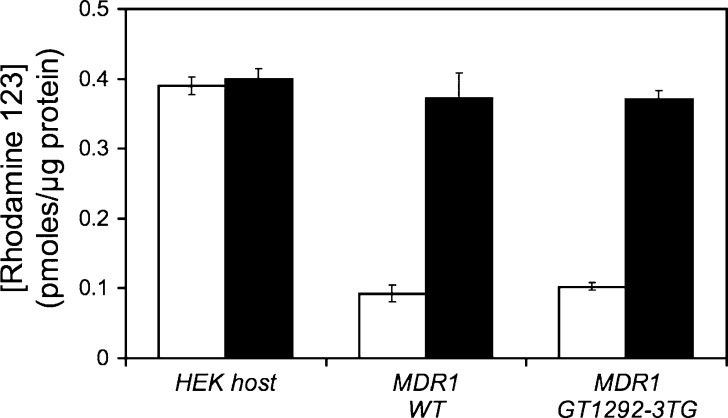
For initial assessment that the expressed P-gp was functional, efflux of R123 was measured after an initial loading of R123 and subsequent incubation in fresh growth media to allow the efflux of R123. These recombinant cells exhibited reduced intracellular accumulation of the P-gp probe substrate R123 and were reversible with addition of a P-gp inhibitor GF120918 (Fig. 2). To evaluate the stability and reproducibility of the recombinant MDR1 cell lines with respect to the functional stability of P-gp expression, we employed doxorubicin as a P-gp substrate and measured the reduction of intracellular levels due to P-gp expression. This assay was performed on cells prior to freezing in liquid N2 for storage and on cells recovered after 4 months in storage. We found that these MDR1 recombinant cells recovered from liquid N2 exhibit similar characteristics in their ability to reduce intracellular concentration of doxorubicin, a P-gp substrate, with no statistical difference. These cells were stable for more than 2 months of passage in culture (Table II).
Fig. 2.
Effects of P-gp inhibitor GF120918 on rhodamine123 efflux in preloaded P-gp recombinant cells. Recombinant cells expressing control and MDR1 variants were seeded into 12-well tissue culture plates in quadruplicate and grown overnight at 37°C. Medium was removed and preincubated with serum-free medium or medium containing 1 μM GF120918 for 5 min before replacing the medium with 1 μM R123 or 1 μM R123 plus 1 μM GF120918 and incubated for 30 min at 37°C. Medium was removed, and the plates were washed with warm PBS. Serum-free medium or medium containing 1 μM GF120918 was added and incubated at 37°C for 30 min. After extensive washing of the cells, cells were lysed with 0.5% DOC. The intracellular R123 fluorescence (λ ex = 485 nm; λ em = 535 nm) was measured on a Victor III multiplate reader. White bars rhodamine123, black bars rhodamine123 + GF120918
Table II.
Stability and Reproducibility of P-gp Function in Parent and MDR1 WT and MDR1 GT1292-3TG Recombinant Cells
| Doxorubicin (percent of HEK host cells) | |||
|---|---|---|---|
| HEK host cells | MDR1 wt (431 Cys) | MDR1 GT1292-3TG (431Leu) | |
| Pre-banked cellsa | 100 ± 2.3 | 61.8 ± 2.8 | 78.3 ± 3.9 |
| 37 days after thawingb | 100 ± 7.5 | 62.9 ± 1.7 | 79.6 ± 1.7 |
| 61 days after thawingb | 100 ± 4.4 | 61.7 ± 4.0 | 80.1 ± 3.7 |
The recombinant cells expressing MDR1 wt or MDR1 GT1292-3TG were exposed to 5 μM doxorubicin for 30 min at 37°C and the doxorubicin concentration were determined. These data were normalized for protein content and expressed as percentage of drug uptake in the HEK host cells. Data expressed were a mean ± SD for quadruplicate
aCells were banked in 10% DMSO in DMEM + 10% FBS growth medium
bCells were thawed and passaged following 4 months of storage in liquid N2
Evaluation of the Functional Impact of the GT1292-3TG Variant on MDR1-Dependent Drug Resistance
A panel of chemotherapeutic agents that included vinblastine, vincristine, doxorubicin, paclitaxel, and topotecan was used to generate dose–response profiles in the recombinant HEK cells. From these profiles, an IC50 value was calculated. As presented in Table III, MDR1GT1292-3TG-expressing cells exhibited increased sensitivity to vinblastine, vincristine, doxorubicin, and paclitaxel compared with MDR1wt, shown by a decrease in drug concentration needed to inhibit growth by 50%.
Table III.
Effects of GT1292-3TG Variation on the Cytotoxicity of Chemotherapeutic Agents in MDR1 Recombinant Cells
| Drug | IC50 (nM) | ||
|---|---|---|---|
| HEK host cells | MDR1 wt (431 Cys) | MDR1 GT1292-3TG (431Leu) | |
| Vinblastine | 6.32 ± 0.795 | 128.0 ± 15.9* | 65.91 ± 5.1*,** |
| Vincristine | 6.69 ± 1.21 | 593.7 ± 61.8* | 307.3 ± 17.0*,** |
| Doxorubicin | 12.75 ± 0.99 | 162.6 ± 17.4* | 37.93 ± 2.6*,** |
| Paclitaxel | 2.35 ± 0.875 | 155.7 ± 27.5* | 87.69 ± 9.2*,** |
| Topotecan | 13.35 ± 3.71 | 42.07 ± 8.5* | 37.36 ± 3.6*,** |
The recombinant cells expressing MDR1 wild type or variants, along with MDR1-negative HEK host cells, were seeded in a 96-well plate. They were exposed to varying concentrations of vincristine, vinblastine, doxorubicin, paclitaxel, or topotecan for 72 h, and cell viability was determined. The dose–response curve for each drug was fitted to estimate IC50 values, as described in “Materials and Methods”. The mean and variations from four replicates were expressed as mean and standard deviation
*p < 0.001 compared with HEK host cells; ** p < 0.001 compared with MDR1 wt recombinant cells
Expression of MDR1wt was able to significantly increase the drug resistance of the HEK host cells doxorubicin 12-fold, vinblastine 20-fold, paclitaxel, 65-fold, and vincristine 88-fold (all p < 0.001). While still significantly higher than the HEK host cells, the IC50 values for recombinant cells expressing MDR1GT1292-3TG were approximately half of that in cells expressing MDR1wt for vinblastine (p < 0.001), vincristine (p < 0.001), and paclitaxel (p < 0.005). Additionally, the IC50 value was decreased by approximately 75% for doxorubicin (p < 0.001). This MDR1-mediated effect was specific in that the control drug substrate topotecan, a predominantly BCRP substrate, and poor P-gp substrate, exhibited a similar degree of sensitivity between the two MDR1-expressing cells.
Intracellular Substrate Accumulation in MDR1wt and MDR1GT1292-3TG Recombinant Cells
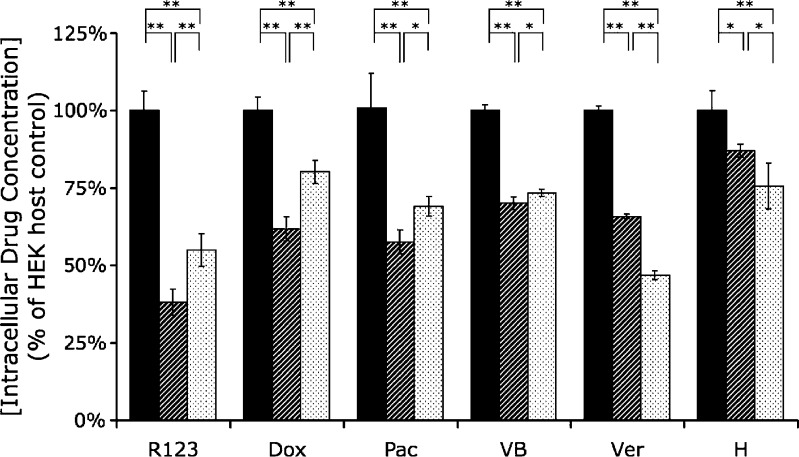
To determine whether MDR1GT1292-3TG efflux transport activity is modulated when compared with wild-type P-gp, we evaluated the steady-state levels of intracellular uptake of R123, doxorubicin, Paclitaxel-Oregon Green® 488, Bodipy FL-vinblastine, Bodipy FL-verapamil, and Hoechst33342 (Fig. 3). MDR1wt-expressing cells showed a significantly greater reduction in drug uptake compared with MDR1GT1292-3TG for R123 (p < 0.01), doxorubicin (p < 0.001), paclitaxel (p < 0.05), and vinblastine (p < 0.05). Conversely, there was a significantly lower intracellular accumulation of verapamil (p < 0.001) and Hoechst33342 (p < 0.05) for MDR1GT1292-3TG compared with MDR1wt (Fig. 3). Collectively, the intracellular uptake of P-gp substrate profile suggests that the MDR1GT1292-3TG variation may increase overall intracellular R123 and cancer drug levels while it reduces the other P-gp substrates Hoechst33342 and verapamil.
Fig. 3.
Comparison of intracellular substrate concentration in cells expressing MDR1 WT and MDR1 GT1292-3TG. Cells were seeded out into 96-well plates and grown overnight. The growth media was then removed and replaced with 1 μM rhodamine123 (R123), 5 μM doxorubicin (Dox), 1 μM Paclitaxel-Oregon Green® 488 (Pac), 1 μM Bodipy FL-Vinblastine (VB), 0.3 μM Bodipy FL-Verapamil (Ver), or 0.4 μM Hoechst33342 (H) in DMEM for 30 min at 37°C. Cells were washed three times with cold PBS and lysed in 0.5% DOC. The respective drug concentrations were determined based on fluorescent intensity measurement and expressed as a percentage of control host HEK cells without MDR1. Results were normalized for protein content. Data expressed are mean and standard deviation of quadruplicate samples. Black columns HEK (−) control, hatched columns MDR1 WT, dotted columns MDR GT1292-3TG. *p < 0.05; **p < 0.01
ATPase Activity
ATPase activity was measured to determine if the change from cysteine to leucine at amino acid 431 in the nucleotide binding domain (NBD) would alter the ability of P-gp to hydrolyze ATP to ADP. We used crude membrane preparations in our assay to determine the vanadate sensitive basel activity. Our results showed that the wild-type and GT1292-3TG variant exhibited comparable vanadate sensitive basal activities of 2.93 ± 0.22 and 2.93 ± 0.34 nmol mg protein−1 min−1, with HEK (−) control cells exhibiting an activity of 1.80 ± 0.21 nmol mg protein−1 min−1.
DISCUSSION
A number of clinically used chemotherapeutic agents are substrates of P-gp, and expression of P-gp in cancer cells and tumors is a primary cause of drug resistance. Interindividual variation in P-gp activity, due to variation in the MDR1 sequence, may influence distribution and delivery of anticancer agents to target cells and tissues. Based on our MDR1 sequence variation analysis, we discovered the novel MDR1GT1292-3TG nucleotide variation that translates to an amino acid substitution from cysteine to leucine at position 431. We have shown that the recombinant cell system expressing MDR1wt,MDR1G1199A, MDR1G1199T, or MDR1G571A is a highly reliable and consistent method suitable for evaluating functional changes in P-gp due to sequence variation (8–10,14).
In this study, we used MDR1GT1292-3TG recombinant HEK cells that express a P-gp variant, with a Cys to Leu substitution at amino acid 431, to evaluate functional changes. Cys431 is located within the conserved Walker A sequence in the NBD. All ABC transporters, including P-gp, have an ATP binding domain, which includes the A-loop, Walker A, Signature, and Walker B conserved sequences (15). The Walker A region spans the eight amino acid sequence “GxxGxGK(S or T)”, where x is any residue (16). Although Cys431 of the Walker A sequence is not highly conserved among ABC transporters, it is conserved in mammalian P-gp (17).
The Walker A sequence is proposed to form a loop that non-covalently binds to the β and γ phosphates of di- and tri-nucleotides, and is essential for ATP hydrolysis. It was proposed that Cys431 and Cys1074 (the corresponding cysteine in the second NBD) were responsible for inhibitory disulfide cross-linking between NBDs in the absence of reducing agents, and addition of ATP was able to protect from this modification (18,19). However, recent data based on structural models predict a 35-Å distance between the two cysteine residues, in the absence of nucleotide, that make disulfide bonding improbable (15,20).
The Cys-less P-gp mutant created by Loo and Clarke replaced the seven endogenous cysteines with alanine and retained its ability to confer resistance to multiple drugs compared with wild type (21). In addition, the Cys431Ala mutation did not impact ATPase activity. When the cysteine was re-inserted at amino acid 431 as the only cysteine within the Cys-less (Cys431Ala) P-gp molecule, covalent modification of the thiol side chain with N-ethylmaleimide inhibited activity of P-gp, suggesting that both NBDs must be active for function (22). In our studies, we also found that both Cys431 and Leu431 (which does not have the ability to form a disulfide bond) exhibit comparable ATPase activity, consistent with the results of the Cys-less mutant study results. Although a direct comparison of Cys431Ala and Cys431Leu with WT P-gp in HEK recombinant cells remained, such a study is beyond the scope of this report. Regardless, mutating amino acids G427, G430, K433, and S434 within the Walker A sequence do modulate ATPase activity (15), to our knowledge, no study shows that mutations to Cys431 result in a drastic change in ATPase activity.
Due to the conservation of Cys431 within mammalian P-gp, and lack of any previously reported genetic variation at Cys431 within the general population, we hypothesized that the GT1292-3TG genetic variation would result in altered function of P-gp. Our cytotoxicity studies showed that Cys431Leu substitution resulted in a significant increase in sensitivity to chemotherapeutic drugs. The largest decrease in drug resistance was found with doxorubicin. Doxorubicin resistance was lowered by greater than 75%, while resistance was lowered by slightly less than half for vinblastine, vincristine, and paclitaxel (Table III).
The significance of estimating functional impacts of genetic variation using stable recombinant cells expressing P-gp and variants should not be underestimated. We first reported the impact of MDR1G1199A genetic variation with recombinant HEK cells to document the increased efflux of chemotherapeutic drugs, resulting in decreased intracellular concentrations of drug, and higher drug resistance in cytotoxicity studies (8). This finding was followed up by Green et al. when they analyzed the MDR1G1199A variation in ovarian cancer patients treated with paclitaxel and carboplatin to evaluate their survival predictive value in vivo (23). Two of the 51 patients they sequenced were heterozygous for the G1199A allele. These two patients’ mean progression free survival was only 2 months as compared with 19 months for the wild-type patients. This was consistent with our prediction that the G1199A variation could potentially decrease the effectiveness of chemotherapy.
However, MDR1 variations, including GT1292-3TG, may also influence other prognostic factors in addition to the disposition of the chemotherapeutic drugs. These factors may be important in the progression of disease. Without subsequent clinical data for a patient with GT1292-3TG, a definitive conclusion cannot be made as to the impact of the variation to clinical response. This is especially true as we found a greater than 2-fold increase in RNA in the GT1292-3TG patient sample. The increased quantity of transcription of the MDR1 gene in this patient, who is heterozygous for the GT1292-3TG and wild-type MDR1, may offset the decreased transport. These and other possibilities remain to be evaluated.
The results of the drug uptake studies in HEK cells correlated well with the resistance data in the cytotoxicity study. The MDR1GT1292-3TG-expressing cells had a higher intracellular accumulation of R123, doxorubicin, paclitaxel, and vinblastine than the wild type, with doxorubicin showing the largest difference (Fig. 3). Although the intracellular concentration of vinblastine in our uptake study was significantly higher in the GT1292-3TG than the wild-type-expressing cells (p < 0.05), their values were very close. This is in comparison with the cytotoxicity data where there is a much larger (~50%) decrease in IC50 from the MDR1wt to the MDR1GT1292-3TG-expressing cells. The uptake assay was performed over a 30-min period, whereas cytotoxicity assay was evaluated over a 4-day period. Therefore, small differences in uptake after a 30-min incubation of vinblastine may not fully predict the magnitude of the cumulative effect of prolonged drug exposure. The decrease in intracellular doxorubicin and paclitaxel in the MDR1-expressing cells were better predictors of resistance. For doxorubicin, the intracellular concentration dropped to 62% of HEK control cells in the MDR1 wild type, with the GT1292-3TG cells only reducing the intracellular by half that amount to 80%. For paclitaxel, the intracellular concentration dropped to 56% of HEK control cells in the MDR1 wild type, with the GT1292-3TG cells reducing the intracellular concentration to 71% of HEK control.
Conversely, for verapamil and Hoechst33342, the intracellular concentration in the MDR1GT1292-3TG-expressing cells was less than the wild type. The substrate-specific differences in intracellular accumulation do not appear to link with ATPase activity. Our studies of the ATPase activity showed no difference between the wild-type P-gp and Cys431Leu variant.
The substrate-specific discrepancy in uptake may be explained by the change in TM domain conformation during the transport cycle (24) together with the presence of multiple drug binding sites within the P-gp protein (25–28). Different conformations of the TM domains of P-gp have been detected by binding ATP, binding AMP·PNP (a non-hydrolyzable ATP analogue), during ATP hydrolysis, and through mutation of the catalytic carboxylates within the NBD (24). Using cysteine-scanning mutagenesis, Loo and Clark showed that the packing of the TM segments was changed when different substrates were bound, revealing different residues to the drug binding pocket (28). It is suggested that P-gp undergoes further conformational changes of the TM domains resulting from ATP hydrolysis, finally resulting in drug transport (29).
There are a number of reported structural models that describe drug binding with varying resolutions and confidence. Garrigues et al. have stated that there are two pharmacophores located very close together that partially overlap (25). Their results strongly suggest verapamil, cyclosporin A, and actinomycin D bind to P-glycoprotein at one site in the binding domain of P-pg; the binding of vinblastine and tentoxin defined another pharmacophore.
Another proposed model for drug binding within the P-gp protein is the “R” and “H” site model (26,27). The R site binds preferentially R123, daunorubicin, doxorubicin, and other anthracyclines, while the H site binds Hoechst33342, quercetin, and colchicines. Additionally, these two sites have approximately equal affinity for vinblastine, etoposide, and dactinomycin (actinomycin D). Doxorubicin and verapamil were shown to be non-competitive inhibitors (30) and thus proposed to bind to differing regions of the P-gp drug binding pocket. Shaprio and Ling (26) proposed that based on their data, and that of Spoelstra et al. (30), verapamil binds preferentially to the H site. In addition, Loo et al. found that the binding sites for verapamil and Hoechst33342 do not overlap with rhodamine in the P-gp drug binding pocket (31). Our data for the uptake studies support these proposals as we have similar substrate groupings as the R and H site model. With the GT1292-3TG variation, when compared with wild type, R123 and doxorubicin have increased uptake, Hoechst and verapamil have decreased uptake, and vinblastine has a minor difference in uptake. Clearly, the impact of GT to TG transition at 1292-3 appeared to reduce efflux transport of substrates within the proposed R site and increase those within the proposed H site. While our results are consistent with the R and H sites model, the impact of the GT1292-3TG variation on the Substrate Induced Fit model, as proposed by Loo et al (28), is not known.
In our MDR1GT1292-3TG variant, there are no amino acid changes within the TM segments; the MDR1GT1292-3TG variation results in a Cys431Leu substitution in the ATP binding domain. Yet, our data suggest that the differences between drugs that had increased transport and decreased transport correlate well with current models of the drug binding pocket. We hypothesize that the Cys431Leu change may affect the communication between the NBD and the TM domains, resulting in altered conformation of the TM segments during ATP binding and/or ATP hydrolysis. This may result in altered drug transport in a substrate-specific manner, affecting each drug binding site in a different way. We believe that this is not due to the loss of the Cys residue, but due to the transition to a larger, hydrophobic Leu residue. These and other possibilities remain to be investigated. Regardless, genetic transition from GT to TG at 1292-3 of MDR1 coding sequence will likely have a significant impact on chemotherapeutic agents that are substrates of P-gp, and the extent and direction of impact appeared to be substrate dependent and may relate to whether the compound binds to different sites within the P-gp drug binding pocket.
It must also be noted that this Cys431Leu variation is the result of two mutations occurring simultaneously at nucleotides 1292 and 1293. Single variation at only 1292 or 1293 has not been reported, nor seen in our patient population. If these mutations were to occur independently of one another, they would result in a Cys431Phe transition (G1292T) or Cys431Trp transition (T1293G). The reported 1292-3 variation found in leukemia subjects are yet to be defined in the healthy general population using a larger sample size. Regardless, if our hypothesis is correct in that the change in P-gp-mediated resistance and uptake is due to the increase in size and hydrophobicity of the resulting amino acid 431 compared with Cys, we believe that the Cys431Phe and Cys431Trp may have similar results to the Cys431Leu transition, and remains to be investigated.
CONCLUSION
We investigated a novel human MDR1GT1292-3TG (Cys431Leu) variation. Using P-gp recombinant cells expressing MDR1WT and MDR1GT1292-3TG, functional impacts of this variant on cytotoxicity and drug uptake were analyzed. Sensitivity to our chemotherapeutic drugs was increased in the MDR1GT1292-3TG-expressing cells, shown by a decrease in IC50 value, when compared with wild-type-expressing cells. Drug uptake studies showed that the effect of the Cys431Leu variation results in a substrate-specific effect where doxorubicin, vinblastine, and paclitaxel had an increased uptake, while verapamil and Hoechst33342 had a decreased uptake compared with wild type. These differences may be due to small steric changes in the NBD that do not have an effect on ATPase activity, but translate to changes in the drug binding pocket, and warrants further research. These data suggest that patients with MDR1GT1292-3TG may be more responsive than their wild-type counterparts, when given chemotherapeutic agents containing doxorubicin, paclitaxel, vinblastine, or vincristine.
Acknowledgements
Supported by NIH grants GM62883, AI077390, AI52663, and NS 39178, and the UW DNA Sequencing and Gene Analysis Center.
References
- 1.Di Nicolantonio F, Mercer SJ, Knight LA, Gabriel FG, Whitehouse PA, Sharma S, et al. Cancer cell adaptation to chemotherapy. BMC Cancer. 2005;5:78. doi: 10.1186/1471-2407-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JH, Yamazaki M. Clinical relevance of P-glycoprotein in drug therapy. Drug Metab Rev. 2003;35(4):417–454. doi: 10.1081/DMR-120026871. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22(47):7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 4.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 5.Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94(8):4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama NN, Kelly EJ, Bui T, Ho RJ. The impact of pharmacologic and genetic knockout of P-glycoprotein on nelfinavir levels in the brain and other tissues in mice. J Pharm Sci. 2005;94(6):1216–1225. doi: 10.1002/jps.20344. [DOI] [PubMed] [Google Scholar]
- 7.Kaddoumi A, Choi SU, Kinman L, Whittington D, Tsai CC, Ho RJ, et al. Inhibition of P-glycoprotein activity at the primate blood–brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35(9):1459–1462. doi: 10.1124/dmd.107.016220. [DOI] [PubMed] [Google Scholar]
- 8.Crouthamel MH, Wu D, Yang Z, Ho RJ. A novel MDR1 G1199T variant alters drug resistance and efflux transport activity of P-glycoprotein in recombinant Hek cells. J Pharm Sci. 2006;95(12):2767–2777. doi: 10.1002/jps.20743. [DOI] [PubMed] [Google Scholar]
- 9.Woodahl EL, Yang Z, Bui T, Shen DD, Ho RJ. Multidrug resistance gene G1199A polymorphism alters efflux transport activity of P-glycoprotein. J Pharmacol Exp Ther. 2004;310(3):1199–1207. doi: 10.1124/jpet.104.065383. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Wu D, Bui T, Ho RJ. A novel human multidrug resistance gene MDR1 variant G571A (G191R) modulates cancer drug resistance and efflux transport. J Pharmacol Exp Ther. 2008;327(2):474–481. doi: 10.1124/jpet.108.138313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambudkar SV, Kim IW, Xia D, Sauna ZE. The A-loop, a novel conserved aromatic acid subdomain upstream of the Walker A motif in ABC transporters, is critical for ATP binding. FEBS Lett. 2006;580(4):1049–1055. doi: 10.1016/j.febslet.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Kim IW, Peng XH, Sauna ZE, FitzGerald PC, Xia D, Muller M, et al. The conserved tyrosine residues 401 and 1044 in ATP sites of human P-glycoprotein are critical for ATP binding and hydrolysis: evidence for a conserved subdomain, the A-loop in the ATP-binding cassette. Biochemistry. 2006;45(24):7605–7616. doi: 10.1021/bi060308o. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Woodahl EL, Wang XY, Bui T, Shen DD, Ho RJ. Semi-quantitative RT-PCR method to estimate full-length mRNA levels of the multidrug resistance gene. Biotechniques. 2002;33(1):196. doi: 10.2144/02331dd03. [DOI] [PubMed] [Google Scholar]
- 14.Woodahl EL, Crouthamel MH, Bui T, Shen DD, Ho RJ. MDR1 (ABCB1) G1199A (Ser400Asn) polymorphism alters transepithelial permeability and sensitivity to anticancer agents. Cancer Chemother Pharmacol. 2009;64(1):183–188. doi: 10.1007/s00280-008-0906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson J, O'Mara ML, Kerr ID. Structure-based interpretation of the mutagenesis database for the nucleotide binding domains of P-glycoprotein. Biochim Biophys Acta. 2008;1778(2):376–391. doi: 10.1016/j.bbamem.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 18.Loo TW, Clarke DM. Drug-stimulated ATPase activity of human P-glycoprotein is blocked by disulfide cross-linking between the nucleotide-binding sites. J Biol Chem. 2000;275(26):19435–19438. doi: 10.1074/jbc.C000222200. [DOI] [PubMed] [Google Scholar]
- 19.Urbatsch IL, Gimi K, Wilke-Mounts S, Lerner-Marmarosh N, Rousseau ME, Gros P, et al. Cysteines 431 and 1074 are responsible for inhibitory disulfide cross-linking between the two nucleotide-binding sites in human P-glycoprotein. J Biol Chem. 2001;276(29):26980–26987. doi: 10.1074/jbc.M010829200. [DOI] [PubMed] [Google Scholar]
- 20.Becker JP, Depret G, Van Bambeke F, Tulkens PM, Prevost M. Molecular models of human P-glycoprotein in two different catalytic states. BMC Struct Biol. 2009;9:3. doi: 10.1186/1472-6807-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem. 1995;270(2):843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- 22.Loo TW, Clarke DM. Covalent modification of human P-glycoprotein mutants containing a single cysteine in either nucleotide-binding fold abolishes drug-stimulated ATPase activity. J Biol Chem. 1995;270(39):22957–22961. doi: 10.1074/jbc.270.39.22957. [DOI] [PubMed] [Google Scholar]
- 23.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97(6):2045–2048. doi: 10.1002/jps.21169. [DOI] [PubMed] [Google Scholar]
- 24.Loo TW, Bartlett MC, Clarke DM. Nucleotide binding, ATP hydrolysis, and mutation of the catalytic carboxylates of human P-glycoprotein cause distinct conformational changes in the transmembrane segments. Biochemistry. 2007;46(32):9328–9336. doi: 10.1021/bi700837y. [DOI] [PubMed] [Google Scholar]
- 25.Garrigues A, Loiseau N, Delaforge M, Ferte J, Garrigos M, Andre F, et al. Characterization of two pharmacophores on the multidrug transporter P-glycoprotein. Mol Pharmacol. 2002;62(6):1288–1298. doi: 10.1124/mol.62.6.1288. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro AB, Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur J Biochem. 1997;250(1):130–137. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro AB, Ling V. The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiol Scand Suppl. 1998;643:227–234. [PubMed] [Google Scholar]
- 28.Loo TW, Bartlett MC, Clarke DM. Substrate-induced conformational changes in the transmembrane segments of human P-glycoprotein. Direct evidence for the substrate-induced fit mechanism for drug binding. J Biol Chem. 2003;278(16):13603–13606. doi: 10.1074/jbc.C300073200. [DOI] [PubMed] [Google Scholar]
- 29.Loo TW, Clarke DM. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J Membr Biol. 2005;206(3):173–185. doi: 10.1007/s00232-005-0792-1. [DOI] [PubMed] [Google Scholar]
- 30.Spoelstra EC, Westerhoff HV, Pinedo HM, Dekker H, Lankelma J. The multidrug-resistance-reverser verapamil interferes with cellular P-glycoprotein-mediated pumping of daunorubicin as a non-competing substrate. Eur J Biochem. 1994;221(1):363–373. doi: 10.1111/j.1432-1033.1994.tb18748.x. [DOI] [PubMed] [Google Scholar]
- 31.Loo TW, Bartlett MC, Clarke DM. Methanethiosulfonate derivatives of rhodamine and verapamil activate human P-glycoprotein at different sites. J Biol Chem. 2003;278(50):50136–50141. doi: 10.1074/jbc.M310448200. [DOI] [PubMed] [Google Scholar]